The Cohesin Release Factor WAPL Restricts Chromatin Loop Extension
- PMID: 28475897
- PMCID: PMC5422210
- DOI: 10.1016/j.cell.2017.04.013
The Cohesin Release Factor WAPL Restricts Chromatin Loop Extension
Abstract
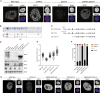
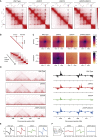
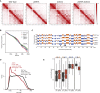
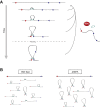
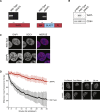
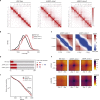
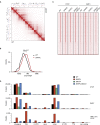
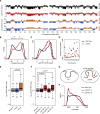
The spatial organization of chromosomes influences many nuclear processes including gene expression. The cohesin complex shapes the 3D genome by looping together CTCF sites along chromosomes. We show here that chromatin loop size can be increased and that the duration with which cohesin embraces DNA determines the degree to which loops are enlarged. Cohesin's DNA release factor WAPL restricts this loop extension and also prevents looping between incorrectly oriented CTCF sites. We reveal that the SCC2/SCC4 complex promotes the extension of chromatin loops and the formation of topologically associated domains (TADs). Our data support the model that cohesin structures chromosomes through the processive enlargement of loops and that TADs reflect polyclonal collections of loops in the making. Finally, we find that whereas cohesin promotes chromosomal looping, it rather limits nuclear compartmentalization. We conclude that the balanced activity of SCC2/SCC4 and WAPL enables cohesin to correctly structure chromosomes.
Keywords: CTCF; MAU2; NIPBL; SCC2; SCC4; TADs; WAPL; chromatin looping; cohesin; loop extrusion.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Blackwood E.M., Kadonaga J.T. Going the distance: a current view of enhancer action. Science. 1998;281:60–63. - PubMed
-
- Blomen V.A., Májek P., Jae L.T., Bigenzahn J.W., Nieuwenhuis J., Staring J., Sacco R., van Diemen F.R., Olk N., Stukalov A. Gene essentiality and synthetic lethality in haploid human cells. Science. 2015;350:1092–1096. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

