Biglycan expression in the melanoma microenvironment promotes invasiveness via increased tissue stiffness inducing integrin-β1 expression
- PMID: 28476030
- PMCID: PMC5522114
- DOI: 10.18632/oncotarget.17160
Biglycan expression in the melanoma microenvironment promotes invasiveness via increased tissue stiffness inducing integrin-β1 expression
Abstract
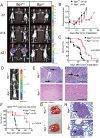
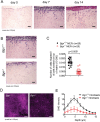
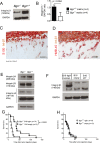
Novel targeted and immunotherapeutic approaches have revolutionized the treatment of metastatic melanoma. A better understanding of the melanoma-microenvironment, in particular the interaction of cells with extracellular matrix molecules, may help to further improve these new therapeutic strategies.We observed that the extracellular matrix molecule biglycan (Bgn) was expressed in certain human melanoma cells and primary fibroblasts when evaluated by microarray-based gene expression analysis. Bgn expression in the melanoma tissues correlated with low overall-survival and low progression-free-survival in patients. To understand the functional role of Bgn we used gene-targeted mice lacking functional Bgn. Here we observed that melanoma growth, metastasis-formation and tumor-related death were reduced in Bgn-/- mice compared to Bgn+/+ mice. In vitro invasion of melanoma cells into organotypic-matrices derived from Bgn-/- fibroblasts was reduced compared to melanoma invasion into Bgn-proficient matrices. Tissue stiffness as determined by atomic-force-microscopy was reduced in Bgn-/- matrices. Isolation of melanoma cells and fibroblasts from the stiffer Bgn+/+ matrices revealed an increase in integrin-β1 expression compared to the Bgn-/- fibroblast matrices. Overexpression of integrin-β1 in B16-melanoma cells abolished the survival benefit seen in Bgn-/- mice. Consistent with the studies performed in mice, the abundance of Bgn-expression in human melanoma samples positively correlated with the expression of integrin-β1, which is in agreement with results from the organotypic invasion-assay and the in vivo mouse studies.This study describes a novel role for Bgn-related tissue stiffness in the melanoma-microenvironment via regulation of integrin-β1 expression by melanoma cells in both mice and humans.
Keywords: biglycan; integrin-β1; melanoma; microenvironment; tissue stiffness.
Conflict of interest statement
The authors have no conflicts of interest to disclose in relation to manuscript.
Figures



References
-
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

