Cell matrix adhesions in cancer: The proteins that form the glue
- PMID: 28476046
- PMCID: PMC5564663
- DOI: 10.18632/oncotarget.17265
Cell matrix adhesions in cancer: The proteins that form the glue
Abstract
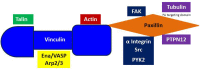
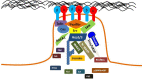
The main purposes of Integrin-mediated cell contacts are to interpret bi-directional signals between the extracellular environment and intracellular proteins, as well as, anchor the cell to a matrix. Many cell adhesion molecules have been discovered with a wide spectrum of responsibilities, including recruiting, activating, elongating, and maintaining. This review will perlustrate some of the key incidences that precede focal adhesion formation. Tyrosine phosphorylation is a key signaling initiation event that leads to the recruitment of multiple proteins to focal adhesion sites. Recruitment and concentration of proteins such as Paxillin and Vinculin to Integrin clutches is necessary for focal adhesion development. The assembled networks are responsible for transmitting signals back and forth from the extracellular matrix (ECM) to Actin and its binding proteins. Cancer cells exhibit highly altered focal adhesion dynamics. This review will highlight some key discoveries in cancer cell adhesion, as well as, identify current gaps in knowledge.
Keywords: cancer; focal adhesions; integrins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Okegawa T, Pong RC, Li Y, Hsieh JT. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta biochimica Polonica. 2004;51:445–457. - PubMed
-
- Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. - PubMed
-
- Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

