Peripheral inflammation affects modulation of nociceptive synaptic transmission in the spinal cord induced by N-arachidonoylphosphatidylethanolamine
- PMID: 28476070
- PMCID: PMC5980568
- DOI: 10.1111/bph.13849
Peripheral inflammation affects modulation of nociceptive synaptic transmission in the spinal cord induced by N-arachidonoylphosphatidylethanolamine
Abstract
Background and purpose: Endocannabinoids play an important role in modulating spinal nociceptive signalling, crucial for the development of pain. The cannabinoid CB1 receptor and the TRPV1 cation channel are both activated by the endocannabinoid anandamide, a product of biosynthesis from the endogenous lipid precursor N-arachidonoylphosphatidylethanolamine (20:4-NAPE). Here, we report CB1 receptor- and TRPV1-mediated effects of 20:4-NAPE on spinal synaptic transmission in control and inflammatory conditions.
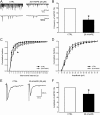
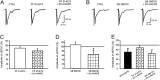
Experimental approach: Spontaneous (sEPSCs) and dorsal root stimulation-evoked (eEPSCs) excitatory postsynaptic currents from superficial dorsal horn neurons in rat spinal cord slices were assessed. Peripheral inflammation was induced by carrageenan. Anandamide concentration was assessed by mass spectrometry.
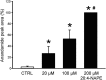
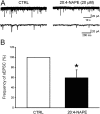
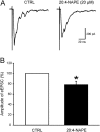
Key results: Application of 20:4-NAPE increased anandamide concentration in vitro. 20:4-NAPE (20 μM) decreased sEPSCs frequency and eEPSCs amplitude in control and inflammatory conditions. The inhibitory effect of 20:4-NAPE was sensitive to CB1 receptor antagonist PF514273 (0.2 μM) in both conditions, but to the TRPV1 antagonist SB366791 (10 μM) only after inflammation. After inflammation, 20:4-NAPE increased sEPSCs frequency in the presence of PF514273 and this increase was blocked by SB366791.
Conclusions and implications: While 20:4-NAPE treatment inhibited the excitatory synaptic transmission in both naive and inflammatory conditions, peripheral inflammation altered the underlying mechanisms. Our data indicate that 20:4-NAPE application induced mainly CB1 receptor-mediated inhibitory effects in naive animals while TRPV1-mediated mechanisms were also involved after inflammation. Increasing anandamide levels for analgesic purposes by applying substrate for its local synthesis may be more effective than systemic anandamide application or inhibition of its degradation.
Linked articles: This article is part of a themed section on Recent Advances in Targeting Ion Channels to Treat Chronic Pain. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.12/issuetoc.
© 2017 The British Pharmacological Society.
Figures



Similar articles
-
Anandamide-Mediated Modulation of Nociceptive Transmission at the Spinal Cord Level.Physiol Res. 2024 Aug 30;73(S1):S435-S448. doi: 10.33549/physiolres.935371. Epub 2024 Jul 2. Physiol Res. 2024. PMID: 38957948 Free PMC article. Review.
-
20:4-NAPE induced changes of mechanical sensitivity and DRG neurons excitability are concentration dependent and mediated via NAPE-PLD.Sci Rep. 2025 Apr 23;15(1):14131. doi: 10.1038/s41598-025-98567-y. Sci Rep. 2025. PMID: 40269193 Free PMC article.
-
Inhibition of synaptic transmission by anandamide precursor 20:4-NAPE is mediated by TRPV1 receptors under inflammatory conditions.Front Mol Neurosci. 2023 Jun 22;16:1188503. doi: 10.3389/fnmol.2023.1188503. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37426071 Free PMC article.
-
Activity of novel lipid glycine transporter inhibitors on synaptic signalling in the dorsal horn of the spinal cord.Br J Pharmacol. 2018 Jun;175(12):2337-2347. doi: 10.1111/bph.14189. Epub 2018 Apr 14. Br J Pharmacol. 2018. PMID: 29500820 Free PMC article.
-
Possible mechanisms of cannabinoid-induced antinociception in the spinal cord.Eur J Pharmacol. 2001 Oct 19;429(1-3):93-100. doi: 10.1016/s0014-2999(01)01309-7. Eur J Pharmacol. 2001. PMID: 11698030 Review.
Cited by
-
Anandamide-Mediated Modulation of Nociceptive Transmission at the Spinal Cord Level.Physiol Res. 2024 Aug 30;73(S1):S435-S448. doi: 10.33549/physiolres.935371. Epub 2024 Jul 2. Physiol Res. 2024. PMID: 38957948 Free PMC article. Review.
-
Hypersensitivity Induced by Intrathecal Bradykinin Administration Is Enhanced by N-oleoyldopamine (OLDA) and Prevented by TRPV1 Antagonist.Int J Mol Sci. 2021 Apr 2;22(7):3712. doi: 10.3390/ijms22073712. Int J Mol Sci. 2021. PMID: 33918267 Free PMC article.
-
Accurate Chemogenetics Determines Electroacupuncture Analgesia Through Increased CB1 to Suppress the TRPV1 Pathway in a Mouse Model of Fibromyalgia.Life (Basel). 2025 May 20;15(5):819. doi: 10.3390/life15050819. Life (Basel). 2025. PMID: 40430245 Free PMC article.
-
20:4-NAPE induced changes of mechanical sensitivity and DRG neurons excitability are concentration dependent and mediated via NAPE-PLD.Sci Rep. 2025 Apr 23;15(1):14131. doi: 10.1038/s41598-025-98567-y. Sci Rep. 2025. PMID: 40269193 Free PMC article.
-
Selective modulation of the cannabinoid type 1 (CB1) receptor as an emerging platform for the treatment of neuropathic pain.Medchemcomm. 2019 Mar 18;10(5):647-659. doi: 10.1039/c8md00595h. eCollection 2019 May 1. Medchemcomm. 2019. PMID: 31191856 Free PMC article. Review.
References
-
- Ahluwalia J, Urban L, Bevan S, Nagy I (2003). Anandamide regulates neuropeptide release from capsaicin‐sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur J Neurosci 17: 2611–2618. - PubMed
-
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I (2000). Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience 100: 685–688. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

