Gamma-glutamyltransferase activity in exosomes as a potential marker for prostate cancer
- PMID: 28476099
- PMCID: PMC5420129
- DOI: 10.1186/s12885-017-3301-x
Gamma-glutamyltransferase activity in exosomes as a potential marker for prostate cancer
Abstract
Background: Exosomes or extracellular vesicles have the potential as a diagnostic marker for various diseases including cancer. In order to identify novel exosomal markers for prostate cancer (PC), we performed proteomic analysis of exosomes isolated from PC cell lines and examined the usefulness of the marker in patients.
Methods: Exosomes isolated by differential centrifugation from the culture medium of androgen-dependent LNCaP prostate cancer cell line and its sublines of partially androgen-independent C4, androgen-independent C4-2 and bone metastatic C4-2B were subjected to iTRAQ-based proteomic analysis. Exosomes were also isolated by immunocapture and separated by size exclusion chromatography and density gradient centrifugation. Protein expression was determined by Western blot analysis. GGT activity was measured using a fluorescent probe, γ-glutamyl hydroxymethyl rhodamine green (gGlu-HMRG). Immunohistochemical analysis of tissues was performed using anti-GGT1 antibody.
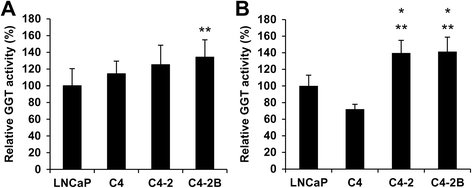
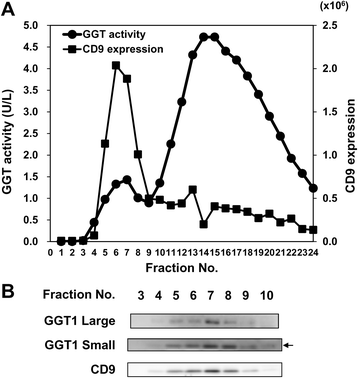
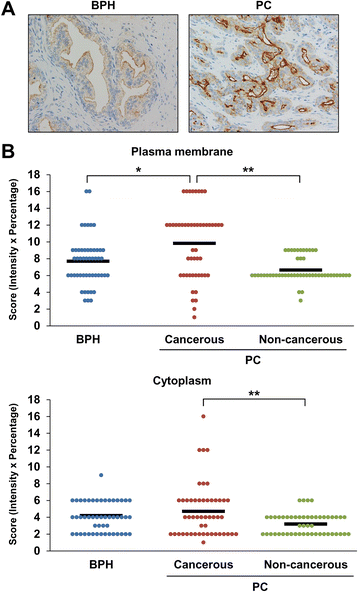
Results: Among proteins upregulated in C4-2 and C4-2B cells than in LNCaP cells, we focused on gamma-glutamyltransferase 1 (GGT1), a cell-surface enzyme that regulates the catabolism of extracellular glutathione. The levels of both GGT1 large and small subunits were elevated in exosomes isolated from C4-2 and C4-2B cells by differential centrifugation and by immunocapture with anti-CD9 or -prostate-specific membrane antigen (PSMA) antibody. In cell lysates and exosomes, GGT1 expression correlated with GGT activity. Size exclusion chromatography of human serum demonstrated the presence of GGT activity and GGT1 subunits in fractions positive for CD9. Density gradient centrifugation revealed the co-presence of GGT1 subunits with CD9 in exosomes isolated by differential centrifugation from human serum. Since GGT activity correlated with GGT1 expression in serum exosomes isolated by differential centrifugation, we measured serum exosomal GGT activity in patients. Unexpectedly, we found that serum exosomal GGT activity was significantly higher in PC patients than in benign prostatic hyperplasia (BPH) patients. In support of this finding, immunohistochemical analysis showed increased GGT1 expression in PC tissues compared with BPH tissues.
Conclusions: Our results suggest that serum exosomal GGT activity could be a useful biomarker for PC.
Keywords: Benign prostatic hyperplasia; Diagnostic marker; Exosome; Prostate cancer; γ-glutamyl transpeptidase; γ-glutamyltransferase 1.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

