Efficacy and safety of rebamipide liquid for chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: a multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II study
- PMID: 28476132
- PMCID: PMC5420134
- DOI: 10.1186/s12885-017-3295-4
Efficacy and safety of rebamipide liquid for chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: a multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II study
Abstract
Background: Recent preclinical and phase I studies have reported that rebamipide decreased the severity of chemoradiotherapy-induced oral mucositis in patients with oral cancer. This placebo-controlled randomized phase II study assessed the clinical benefit of rebamipide in reducing the incidence of severe chemoradiotherapy-induced oral mucositis in patients with head and neck cancer (HNC).
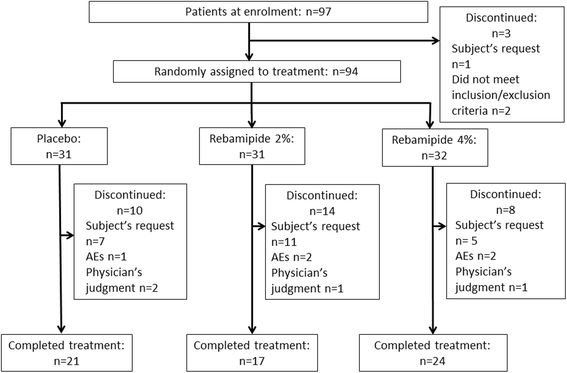
Methods: Patients aged 20-75 years with HNC who were scheduled to receive chemoradiotherapy were enrolled. Patients were randomized to receive rebamipide 2% liquid, rebamipide 4% liquid, or placebo. The primary endpoint was the incidence of grade ≥ 3 oral mucositis determined by clinical examination and assessed by central review according to the Common Terminology Criteria of Adverse Events version 3.0. Secondary endpoints were the time to onset of grade ≥ 3 oral mucositis and the incidence of functional impairment (grade ≥ 3) based on the evaluation by the Oral Mucositis Evaluation Committee.
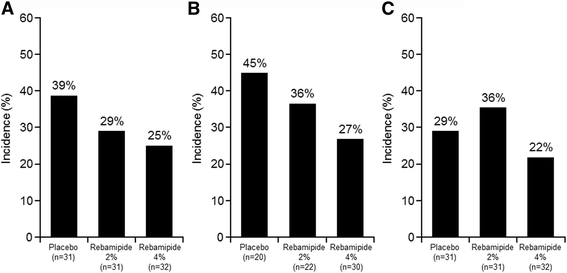
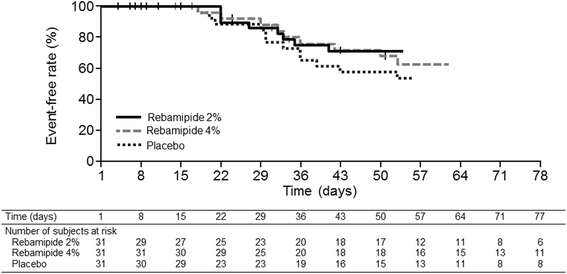
Results: From April 2014 to August 2015, 97 patients with HNC were enrolled, of whom 94 received treatment. The incidence of grade ≥ 3 oral mucositis was 29% and 25% in the rebamipide 2% and 4% groups, respectively, compared with 39% in the placebo group. The proportion of patients who did not develop grade ≥ 3 oral mucositis by day 50 of treatment was 57.9% in the placebo group, whereas the proportion was 68.0% in the rebamipide 2% group and 71.3% in the rebamipide 4% group. The incidences of adverse events potentially related to the study drug were 16%, 26%, and 13% in the placebo, rebamipide 2%, and rebamipide 4% groups, respectively. There was no significant difference in treatment compliance among the groups.
Conclusions: The present phase II study suggests that mouth washing with rebamipide may be effective and safe for patients with HNC receiving chemoradiotherapy, and 4% liquid is the optimal dose of rebamipide.
Trial registration: ClinicalTrials.gov under the identifier NCT02085460 (the date of trial registration: March 11, 2014).
Keywords: Chemoradiotherapy; Head and neck cancer; Oral mucositis; Placebo-controlled; Randomized; Rebamipide liquid.
Figures
Similar articles
-
The post hoc analysis comparing the severity grades of chemoradiotherapy-induced oral mucositis scored between the central and local assessors in a multicenter, randomized controlled trial of rebamipide for head and neck cancer.Int J Clin Oncol. 2019 Mar;24(3):241-247. doi: 10.1007/s10147-018-1355-7. Epub 2018 Nov 13. Int J Clin Oncol. 2019. PMID: 30426268 Free PMC article. Clinical Trial.
-
Rebamipide gargle in preventive management of chemo-radiotherapy induced oral mucositis.Oral Oncol. 2017 Sep;72:179-182. doi: 10.1016/j.oraloncology.2017.07.024. Epub 2017 Jul 28. Oral Oncol. 2017. PMID: 28797456 Clinical Trial.
-
Rebamipide gargle and benzydamine gargle in prevention and management of chemo-radiotherapy and radiotherapy-induced oral mucositis in head and neck cancer patients (randomized clinical trial).BMC Oral Health. 2024 Jun 1;24(1):645. doi: 10.1186/s12903-024-04379-3. BMC Oral Health. 2024. PMID: 38824583 Free PMC article. Clinical Trial.
-
Effects of honey on oral mucositis in patients with head and neck cancer: A meta-analysis.Laryngoscope. 2015 Sep;125(9):2085-92. doi: 10.1002/lary.25233. Epub 2015 Mar 16. Laryngoscope. 2015. PMID: 25778825 Review.
-
Does nutritional support prevent severe mucositis in patients with head and neck cancer treated with chemoradiotherapy? A systematic review and meta-analysis.Clin Nutr ESPEN. 2025 Apr;66:547-555. doi: 10.1016/j.clnesp.2025.02.007. Epub 2025 Feb 13. Clin Nutr ESPEN. 2025. PMID: 39954955
Cited by
-
The effectiveness of mouthwashes in alleviating radiation-induced oral mucositis in head and neck cancer patients: a systematic review.Oral Radiol. 2019 Sep;35(3):207-223. doi: 10.1007/s11282-018-0361-9. Epub 2018 Dec 10. Oral Radiol. 2019. PMID: 30535943
-
Hydrogel Formulations Incorporating Drug Nanocrystals Enhance the Therapeutic Effect of Rebamipide in a Hamster Model for Oral Mucositis.Pharmaceutics. 2020 Jun 9;12(6):532. doi: 10.3390/pharmaceutics12060532. Pharmaceutics. 2020. PMID: 32527029 Free PMC article.
-
The effectiveness of rebamipide mouthwash therapy for radiotherapy and chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: a systematic review and meta-analysis.J Pharm Health Care Sci. 2019 Jul 25;5:16. doi: 10.1186/s40780-019-0146-2. eCollection 2019. J Pharm Health Care Sci. 2019. PMID: 31367460 Free PMC article.
-
The post hoc analysis comparing the severity grades of chemoradiotherapy-induced oral mucositis scored between the central and local assessors in a multicenter, randomized controlled trial of rebamipide for head and neck cancer.Int J Clin Oncol. 2019 Mar;24(3):241-247. doi: 10.1007/s10147-018-1355-7. Epub 2018 Nov 13. Int J Clin Oncol. 2019. PMID: 30426268 Free PMC article. Clinical Trial.
-
Expert consensus on the prevention and treatment of radiochemotherapy-induced oral mucositis.Int J Oral Sci. 2025 Jul 15;17(1):54. doi: 10.1038/s41368-025-00382-8. Int J Oral Sci. 2025. PMID: 40664676 Free PMC article.
References
-
- Datamonitor Healthcare Market Briefs Product Code: BFHC0606: Oral Mucositis Challenging the Priorities of Cancer Therapy.
-
- Le QT, Kim HE, Schneider CJ, Muraközy G, Skladowski K, Reinisch S, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo-controlled study. J Clin Oncol. 2011;29:2808–2814. doi: 10.1200/JCO.2010.32.4095. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

