Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity
- PMID: 28476157
- PMCID: PMC5418760
- DOI: 10.1186/s12974-017-0871-0
Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity
Abstract
Background: As the primary immune response cell in the central nervous system, microglia constantly monitor the microenvironment and respond rapidly to stress, infection, and injury, making them important modulators of neuroinflammatory responses. In diseases such as Parkinson's disease, Alzheimer's disease, multiple sclerosis, and human immunodeficiency virus-induced dementia, activation of microglia precedes astrogliosis and overt neuronal loss. Although microgliosis is implicated in manganese (Mn) neurotoxicity, the role of microglia and glial crosstalk in Mn-induced neurodegeneration is poorly understood.
Methods: Experiments utilized immunopurified murine microglia and astrocytes using column-free magnetic separation. The effect of Mn on microglia was investigated using gene expression analysis, Mn uptake measurements, protein production, and changes in morphology. Additionally, gene expression analysis was used to determine the effect Mn-treated microglia had on inflammatory responses in Mn-exposed astrocytes.
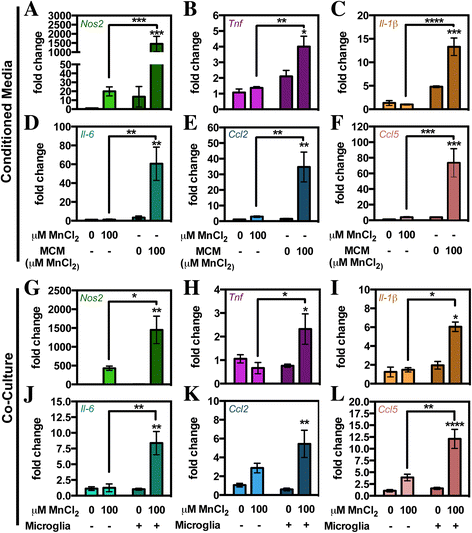
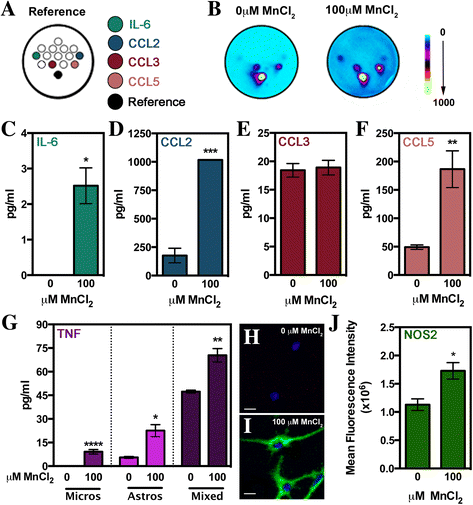
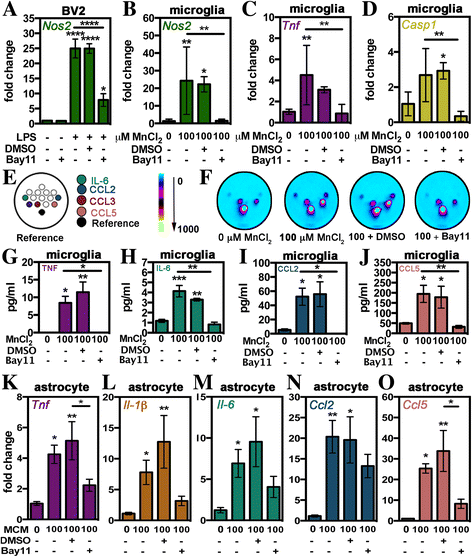
Results: Immunofluorescence and flow cytometric analysis of immunopurified microglia and astrocytes indicated cultures were 97 and 90% pure, respectively. Mn treatment in microglia resulted in a dose-dependent increase in pro-inflammatory gene expression, transition to a mixed M1/M2 phenotype, and a de-ramified morphology. Conditioned media from Mn-exposed microglia (MCM) dramatically enhanced expression of mRNA for Tnf, Il-1β, Il-6, Ccl2, and Ccl5 in astrocytes, as did exposure to Mn in the presence of co-cultured microglia. MCM had increased levels of cytokines and chemokines including IL-6, TNF, CCL2, and CCL5. Pharmacological inhibition of NF-κB in microglia using Bay 11-7082 completely blocked microglial-induced astrocyte activation, whereas siRNA knockdown of Tnf in primary microglia only partially inhibited neuroinflammatory responses in astrocytes.
Conclusions: These results provide evidence that NF-κB signaling in microglia plays an essential role in inflammatory responses in Mn toxicity by regulating cytokines and chemokines that amplify the activation of astrocytes.
Keywords: Glial crosstalk; Manganism; NF-κB; Neuroinflammation; Tumor necrosis factor.
Figures





References
-
- Neal AP, Guilarte TR. Mechanisms of heavy metal neurotoxicity: lead and manganese. J Drug Metab Toxicol S. 2012;5:2.
-
- Huang C-C. Parkinsonism induced by chronic manganese intoxication—an experience in Taiwan. Chang Gung Med J. 2007;30:385–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

