Wnt/beta-catenin pathway: modulating anticancer immune response
- PMID: 28476164
- PMCID: PMC5420131
- DOI: 10.1186/s13045-017-0471-6
Wnt/beta-catenin pathway: modulating anticancer immune response
Abstract
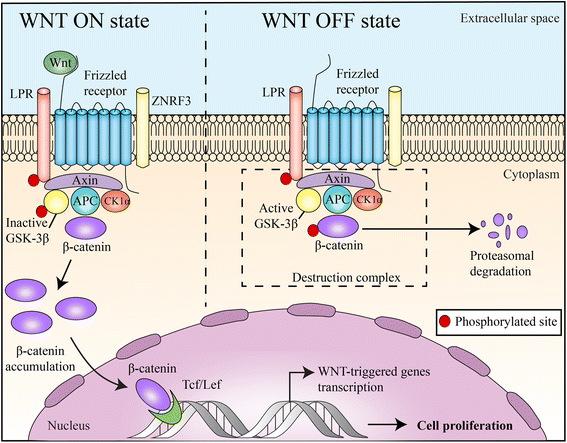
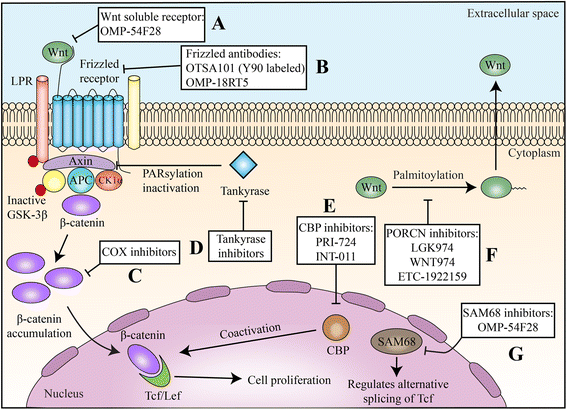
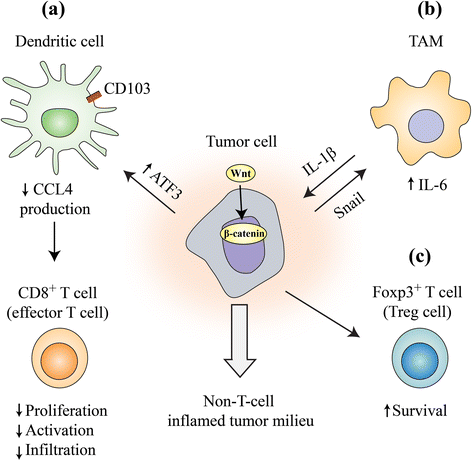
Wnt/β-catenin signaling, a highly conserved pathway through evolution, regulates key cellular functions including proliferation, differentiation, migration, genetic stability, apoptosis, and stem cell renewal. The Wnt pathway mediates biological processes by a canonical or noncanonical pathway, depending on the involvement of β-catenin in signal transduction. β-catenin is a core component of the cadherin protein complex, whose stabilization is essential for the activation of Wnt/β-catenin signaling. As multiple aberrations in this pathway occur in numerous cancers, WNT-directed therapy represents an area of significant developmental therapeutics focus. The recently described role of Wnt/β-catenin pathway in regulating immune cell infiltration of the tumor microenvironment renewed the interest, given its potential impact on responses to immunotherapy treatments. This article summarizes the role of Wnt/β-catenin pathway in cancer and ongoing therapeutic strategies involving this pathway.
Keywords: Cancer immune regulation; Immune exclusion; Immunotherapy; Wnt; β-catenin.
Figures
Similar articles
-
Wnt pathway modulators in cancer therapeutics: An update on completed and ongoing clinical trials.Int J Cancer. 2022 Mar 1;150(5):727-740. doi: 10.1002/ijc.33811. Epub 2021 Oct 11. Int J Cancer. 2022. PMID: 34536299 Review.
-
Perspectives on the role of Wnt biology in cancer.Sci Signal. 2019 Jul 9;12(589):eaay4494. doi: 10.1126/scisignal.aay4494. Sci Signal. 2019. PMID: 31289213
-
The WNT/β-catenin signaling inhibitor XAV939 enhances the elimination of LNCaP and PC-3 prostate cancer cells by prostate cancer patient lymphocytes in vitro.Sci Rep. 2019 Mar 18;9(1):4761. doi: 10.1038/s41598-019-41182-5. Sci Rep. 2019. PMID: 30886380 Free PMC article.
-
Wnt signaling in osteosarcoma.Adv Exp Med Biol. 2014;804:33-45. doi: 10.1007/978-3-319-04843-7_2. Adv Exp Med Biol. 2014. PMID: 24924167 Review.
-
Role of Wnt/β-catenin signaling in drug resistance of pancreatic cancer.Curr Pharm Des. 2012;18(17):2464-71. doi: 10.2174/13816128112092464. Curr Pharm Des. 2012. PMID: 22372504 Review.
Cited by
-
Influence of the Microbiome Metagenomics and Epigenomics on Gastric Cancer.Int J Mol Sci. 2022 Nov 9;23(22):13750. doi: 10.3390/ijms232213750. Int J Mol Sci. 2022. PMID: 36430229 Free PMC article. Review.
-
Baseline Hedgehog Pathway Activation and Increase of Plasma Wnt1 Protein Are Associated with Resistance to Immune Checkpoint Inhibitors in Advanced Non-Small-Cell Lung Cancer.Cancers (Basel). 2021 Mar 5;13(5):1107. doi: 10.3390/cancers13051107. Cancers (Basel). 2021. PMID: 33807552 Free PMC article.
-
Single-cell and spatial sequencing identifies senescent and germinal tumor cells in adamantinomatous craniopharyngiomas.Cell Biosci. 2024 Sep 2;14(1):112. doi: 10.1186/s13578-024-01299-1. Cell Biosci. 2024. PMID: 39223689 Free PMC article.
-
Salicylanilides and Their Anticancer Properties.Int J Mol Sci. 2023 Jan 15;24(2):1728. doi: 10.3390/ijms24021728. Int J Mol Sci. 2023. PMID: 36675241 Free PMC article. Review.
-
Utilizing precision medicine to modulate the prostate tumor microenvironment and enhance immunotherapy.Urol Oncol. 2019 Aug;37(8):535-542. doi: 10.1016/j.urolonc.2018.11.009. Epub 2018 Nov 29. Urol Oncol. 2019. PMID: 30503851 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

