Podocytes regulate the glomerular basement membrane protein nephronectin by means of miR-378a-3p in glomerular diseases
- PMID: 28476557
- PMCID: PMC5658661
- DOI: 10.1016/j.kint.2017.03.005
Podocytes regulate the glomerular basement membrane protein nephronectin by means of miR-378a-3p in glomerular diseases
Abstract
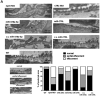
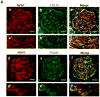
The pathophysiology of many proteinuric kidney diseases is poorly understood, and microRNAs (miRs) regulation of these diseases has been largely unexplored. Here, we tested whether miR-378a-3p is a novel regulator of glomerular diseases. MiR-378a-3p has two predicted targets relevant to glomerular function, the glomerular basement membrane matrix component, nephronectin (NPNT), and vascular endothelial growth factor VEGF-A. In zebrafish (Danio rerio), miR-378a-3p mimic injection or npnt knockdown by a morpholino oligomer caused an identical phenotype consisting of edema, proteinuria, podocyte effacement, and widening of the glomerular basement membrane in the lamina rara interna. Zebrafish vegf-A protein could not rescue this phenotype. However, mouse Npnt constructs containing a mutated 3'UTR region prevented the phenotype caused by miR-378a-3p mimic injection. Overexpression of miR-378a-3p in mice confirmed glomerular dysfunction in a mammalian model. Biopsies from patients with focal segmental glomerulosclerosis and membranous nephropathy had increased miR-378a-3p expression and reduced glomerular levels of NPNT. Thus, miR-378a-3p-mediated suppression of the glomerular matrix protein NPNT is a novel mechanism for proteinuria development in active glomerular diseases.
Keywords: glomerular basement membrane; membranous glomerulonephropathy; microRNA; nephronectin; podocytes.
Copyright © 2017 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures










Comment in
-
Foreseeing the future of glomerular disease through slits: miR-NPNT axis.Kidney Int. 2017 Oct;92(4):782-784. doi: 10.1016/j.kint.2017.04.036. Kidney Int. 2017. PMID: 28938947
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

