A multi-laboratory study of diverse RSV neutralization assays indicates feasibility for harmonization with an international standard
- PMID: 28476625
- PMCID: PMC5439532
- DOI: 10.1016/j.vaccine.2017.04.053
A multi-laboratory study of diverse RSV neutralization assays indicates feasibility for harmonization with an international standard
Abstract
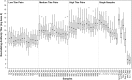
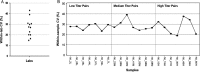
A current barrier to the standardized evaluation of respiratory syncytial virus (RSV) vaccine candidates is the wide variety of virus neutralization assay formats currently in use for assessing immunogenicity. Assay formats vary widely in labor intensiveness, duration, and sample throughput. Furthermore, the cell lines and virus strains used are not consistent among formats. The purpose of this multi-laboratory study was to assess the variability across a diverse array of assay formats that quantitate RSV neutralizing antibodies. Using a common specimen panel, the degree of overall agreement among existing assays was evaluated to inform on the need for harmonization of assay results. A total of 12 laboratories participated in the blinded survey study by testing a panel comprised of 57 samples chosen to span the reportable titer range of the assays. An independent statistical analysis was conducted to measure overall agreement of assay results. This analysis showed that precision was consistently high, whereas agreement varied widely among assays. To examine whether agreement could be improved, we conducted a harmonization exercise using a variety of sample types as pseudo standards. The results showed that the level of agreement could be improved, and provided information on the suitability of samples for developing an international standard.
Keywords: Antibodies; Assay; International standard; Neutralization; Respiratory syncytial virus; Vaccine.
Copyright © 2017. Published by Elsevier Ltd.
Figures


References
-
- Langley G.F., Anderson L.J. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J. 2011;30(6):510–517. PMID: 21487331. - PubMed
-
- Hall C.B. The burgeoning burden of respiratory syncytial virus among children. Infect Disord Drug Targets. 2012;12(2):92–97. PMID: 22335498. - PubMed
-
- Glezen W.P., Taber L.H., Frank A.L., Kasel J.A. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–546. PMID: 3706232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

