Biosynthesis of the Pharmaceutically Important Fungal Ergot Alkaloid Dihydrolysergic Acid Requires a Specialized Allele of cloA
- PMID: 28476772
- PMCID: PMC5494617
- DOI: 10.1128/AEM.00805-17
Biosynthesis of the Pharmaceutically Important Fungal Ergot Alkaloid Dihydrolysergic Acid Requires a Specialized Allele of cloA
Abstract
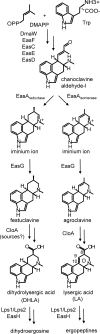
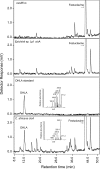
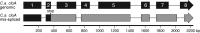
Ergot alkaloids are specialized fungal metabolites that are important as the bases of several pharmaceuticals. Many ergot alkaloids are derivatives of lysergic acid (LA) and have vasoconstrictive activity, whereas several dihydrolysergic acid (DHLA) derivatives are vasorelaxant. The pathway to LA is established, with the P450 monooxygenase CloA playing a key role in oxidizing its substrate agroclavine to LA. We analyzed the activities of products of cloA alleles from different fungi relative to DHLA biosynthesis by expressing them in a mutant of the fungus Neosartorya fumigata that accumulates festuclavine, the precursor to DHLA. Transformants expressing CloA from Epichloë typhina × Epichloë festucae, which oxidizes agroclavine to LA, failed to oxidize festuclavine to DHLA. In substrate feeding experiments, these same transformants oxidized exogenously supplied agroclavine to LA, indicating that a functional CloA was produced. A genomic clone of cloA from Claviceps africana, a sorghum ergot fungus that produces a DHLA derivative, was cloned and expressed in the festuclavine-accumulating mutant of N. fumigata, but several introns in this genomic clone were not processed properly. Expression of a synthetic intron-free version of C. africanacloA resulted in the accumulation of DHLA as assessed by fluorescence high-pressure liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS). In substrate feeding experiments, the C. africana CloA also accepted agroclavine as the substrate, oxidizing it to LA. The data indicate that a specialized allele of cloA is required for DHLA biosynthesis and that the pharmaceutically important compound DHLA can be produced in engineered N. fumigataIMPORTANCE Ergot alkaloids are fungal metabolites that have impacted humankind historically as poisons and more recently as pharmaceuticals used to treat dementia, migraines, and other disorders. Much is known about the biosynthesis of ergot alkaloids that are derived from lysergic acid (LA), but important questions remain about a parallel pathway to ergot alkaloids derived from dihydrolysergic acid (DHLA). DHLA-derived alkaloids have minor structural differences compared to LA-derived alkaloids but can have very different activities. To understand how DHLA is made, we analyzed activities of a key enzyme in the DHLA pathway and found that it differed from its counterpart in the LA pathway. Our data indicate a critical difference between the two pathways and provide a strategy for producing DHLA by modifying a model fungus. The ability to produce DHLA in a model fungus may facilitate synthesis of DHLA-derived pharmaceuticals.
Keywords: P450 monooxygenase; dihydrolysergic acid; ergot alkaloids; lysergic acid.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
Ergot Alkaloid Biosynthesis in the Maize (Zea mays) Ergot Fungus Claviceps gigantea.J Agric Food Chem. 2017 Dec 13;65(49):10703-10710. doi: 10.1021/acs.jafc.7b04272. Epub 2017 Dec 5. J Agric Food Chem. 2017. PMID: 29172518 Free PMC article.
-
Heterologous expression of lysergic acid and novel ergot alkaloids in Aspergillus fumigatus.Appl Environ Microbiol. 2014 Oct;80(20):6465-72. doi: 10.1128/AEM.02137-14. Epub 2014 Aug 8. Appl Environ Microbiol. 2014. PMID: 25107976 Free PMC article.
-
Genetic Reprogramming of the Ergot Alkaloid Pathway of Metarhizium brunneum.Appl Environ Microbiol. 2020 Sep 17;86(19):e01251-20. doi: 10.1128/AEM.01251-20. Print 2020 Sep 17. Appl Environ Microbiol. 2020. PMID: 32769181 Free PMC article.
-
Ergot alkaloids--biology and molecular biology.Alkaloids Chem Biol. 2006;63:45-86. doi: 10.1016/s1099-4831(06)63002-2. Alkaloids Chem Biol. 2006. PMID: 17133714 Review.
-
Clavine Alkaloids Gene Clusters of Penicillium and Related Fungi: Evolutionary Combination of Prenyltransferases, Monooxygenases and Dioxygenases.Genes (Basel). 2017 Nov 24;8(12):342. doi: 10.3390/genes8120342. Genes (Basel). 2017. PMID: 29186777 Free PMC article. Review.
Cited by
-
Ergot Alkaloid Biosynthesis in the Maize (Zea mays) Ergot Fungus Claviceps gigantea.J Agric Food Chem. 2017 Dec 13;65(49):10703-10710. doi: 10.1021/acs.jafc.7b04272. Epub 2017 Dec 5. J Agric Food Chem. 2017. PMID: 29172518 Free PMC article.
-
A Major Facilitator Superfamily Transporter Contributes to Ergot Alkaloid Accumulation but Not Secretion in Aspergillus leporis.Appl Microbiol (Basel). 2024 Mar;4(1):406-417. doi: 10.3390/applmicrobiol4010028. Epub 2024 Feb 20. Appl Microbiol (Basel). 2024. PMID: 39055383 Free PMC article.
-
Independent Evolution of a Lysergic Acid Amide in Aspergillus Species.Appl Environ Microbiol. 2021 Nov 24;87(24):e0180121. doi: 10.1128/AEM.01801-21. Epub 2021 Sep 29. Appl Environ Microbiol. 2021. PMID: 34586904 Free PMC article.
-
Dihydroergotamine and Bromocriptine: Potential Drugs for the Treatment of Major Depressive Disorder and Alzheimer's Disease Comorbidity.Mol Neurobiol. 2025 Feb;62(2):2493-2514. doi: 10.1007/s12035-024-04416-w. Epub 2024 Aug 12. Mol Neurobiol. 2025. PMID: 39134826
-
A Baeyer-Villiger Monooxygenase Gene Involved in the Synthesis of Lysergic Acid Amides Affects the Interaction of the Fungus Metarhizium brunneum with Insects.Appl Environ Microbiol. 2021 Aug 11;87(17):e0074821. doi: 10.1128/AEM.00748-21. Epub 2021 Aug 11. Appl Environ Microbiol. 2021. PMID: 34160271 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

