Mutating a conserved cysteine in GPIHBP1 reduces amounts of GPIHBP1 in capillaries and abolishes LPL binding
- PMID: 28476858
- PMCID: PMC5496041
- DOI: 10.1194/jlr.M076943
Mutating a conserved cysteine in GPIHBP1 reduces amounts of GPIHBP1 in capillaries and abolishes LPL binding
Abstract
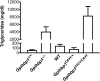
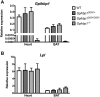
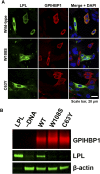
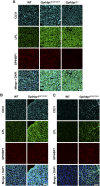
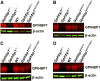
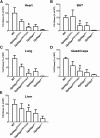
Mutation of conserved cysteines in proteins of the Ly6 family cause human disease-chylomicronemia in the case of glycosylphosphatidylinositol-anchored HDL binding protein 1 (GPIHBP1) and paroxysmal nocturnal hemoglobinuria in the case of CD59. A mutation in a conserved cysteine in CD59 prevented the protein from reaching the surface of blood cells. In contrast, mutation of conserved cysteines in human GPIHBP1 had little effect on GPIHBP1 trafficking to the surface of cultured CHO cells. The latter findings were somewhat surprising and raised questions about whether CHO cell studies accurately model the fate of mutant GPIHBP1 proteins in vivo. To explore this concern, we created mice harboring a GPIHBP1 cysteine mutation (p.C63Y). The p.C63Y mutation abolished the ability of mouse GPIHBP1 to bind LPL, resulting in severe chylomicronemia. The mutant GPIHBP1 was detectable by immunohistochemistry on the surface of endothelial cells, but the level of expression was ∼70% lower than in WT mice. The mutant GPIHBP1 protein in mouse tissues was predominantly monomeric. We conclude that mutation of a conserved cysteine in GPIHBP1 abolishes the ability of GPIHBP1 to bind LPL, resulting in mislocalization of LPL and severe chylomicronemia. The mutation reduced but did not eliminate GPIHBP1 on the surface of endothelial cells in vivo.
Keywords: chylomicrons; endothelial cells; glycosylphosphatidylinositol-anchored HDL binding protein 1; lipids/chemistry; lipolysis and fatty acid metabolism; lipoprotein lipase; triglycerides.
Copyright © 2017 by the American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors have no financial interests to declare.
Figures


References
-
- Beigneux A. P., Davies B., Gin P., Weinstein M. M., Farber E., Qiao X., Peale P., Bunting S., Walzem R. L., Wong J. S., et al. . 2007. Glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 5: 279–291. - PMC - PubMed
-
- Mysling S., Kristensen K. K., Larsson M., Beigneux A. P., Gardsvoll H., Fong L. G., Bensadouen A., Jorgensen T. J., Young S. G., and Ploug M.. 2016. The acidic domain of the endothelial membrane protein GPIHBP1 stabilizes lipoprotein lipase activity by preventing unfolding of its catalytic domain. eLife. 5: e12095. - PMC - PubMed
-
- Fry B. G., Wuster W., Kini R. M., Brusic V., Khan A., Venkataraman D., and Rooney A. P.. 2003. Molecular evolution and phylogeny of elapid snake venom three-finger toxins. J. Mol. Evol. 57: 110–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

