The long non-coding RNA HOTAIR enhances pancreatic cancer resistance to TNF-related apoptosis-inducing ligand
- PMID: 28476883
- PMCID: PMC5481552
- DOI: 10.1074/jbc.M117.786830
The long non-coding RNA HOTAIR enhances pancreatic cancer resistance to TNF-related apoptosis-inducing ligand
Abstract
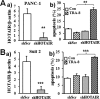
Pancreatic cancer is a malignant neoplasm with a high mortality rate. Therapeutic agents that activate TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis have shown promising efficacy, but many pancreatic cancers are resistant to TRAIL therapy. Epigenetic regulation plays important roles in tumor pathogenesis and resistance, and a recent study indicated that the long non-coding RNA HOX transcript antisense RNA (HOTAIR) is overexpressed in pancreatic cancer. However, the role of HOTAIR in pancreatic cancer resistance to anticancer agents is unknown. The present study determined the role of HOTAIR in pancreatic cancer TRAIL resistance and investigated the underlying molecular mechanisms. We observed that TRAIL-resistant pancreatic cancer cells had higher levels of HOTAIR expression, whereas TRAIL-sensitive pancreatic cancer cells had lower HOTAIR levels. Overexpressing HOTAIR in TRAIL-sensitive cells attenuated TRAIL-induced apoptosis, and shRNA-mediated HOTAIR knockdown in TRAIL-resistant PANC-1 cells sensitized them to TRAIL-induced apoptosis. These results support a causative effect of HOTAIR on TRAIL sensitivity. Mechanistically, we found that increased HOTAIR expression inhibited the expression of the TRAIL receptor death receptor 5 (DR5), whereas HOTAIR knockdown increased DR5 expression. We further demonstrated that HOTAIR regulates DR5 expression via the epigenetic regulator enhancer of zeste homolog 2 (EZH2) and that EZH2 controls histone H3 lysine 27 trimethylation on the DR5 gene. Taken together, these results demonstrate that high HOTAIR levels increase the resistance of pancreatic cancer cells to TRAIL-induced apoptosis via epigenetic regulation of DR5 expression. Our study therefore supports the notion that targeting HOTAIR function may represent a strategy to overcome TRAIL resistance in pancreatic cancer.
Keywords: DR5; TRAIL; apoptosis; death receptor 5; histone methylation; long non-coding RNA (long ncRNA, lncRNA); pancreatic cancer.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





Similar articles
-
HOTAIR-mediated reciprocal regulation of EZH2 and DNMT1 contribute to polyphyllin I-inhibited growth of castration-resistant prostate cancer cells in vitro and in vivo.Biochim Biophys Acta Gen Subj. 2018 Mar;1862(3):589-599. doi: 10.1016/j.bbagen.2017.12.001. Epub 2017 Dec 6. Biochim Biophys Acta Gen Subj. 2018. PMID: 29221985
-
The herbal compound cryptotanshinone restores sensitivity in cancer cells that are resistant to the tumor necrosis factor-related apoptosis-inducing ligand.J Biol Chem. 2013 Oct 11;288(41):29923-33. doi: 10.1074/jbc.M113.483909. Epub 2013 Aug 28. J Biol Chem. 2013. PMID: 23986445 Free PMC article.
-
DR4 specific TRAIL variants are more efficacious than wild-type TRAIL in pancreatic cancer.Cancer Biol Ther. 2014;15(12):1658-66. doi: 10.4161/15384047.2014.972183. Cancer Biol Ther. 2014. PMID: 25482930 Free PMC article.
-
Targeting miRNAs associated with surface expression of death receptors to modulate TRAIL resistance in breast cancer.Cancer Lett. 2016 Dec 28;383(2):154-160. doi: 10.1016/j.canlet.2016.09.021. Epub 2016 Sep 28. Cancer Lett. 2016. PMID: 27693456 Review.
-
Long non-coding RNA HOTAIR in carcinogenesis and metastasis.Acta Biochim Biophys Sin (Shanghai). 2014 Jan;46(1):1-5. doi: 10.1093/abbs/gmt117. Epub 2013 Oct 27. Acta Biochim Biophys Sin (Shanghai). 2014. PMID: 24165275 Free PMC article. Review.
Cited by
-
Long noncoding RNAs: role and contribution in pancreatic cancer.Transcription. 2021 Feb;12(1):12-27. doi: 10.1080/21541264.2021.1922071. Epub 2021 May 26. Transcription. 2021. PMID: 34036896 Free PMC article. Review.
-
LncRNA HOTAIR: A Potential Prognostic Factor and Therapeutic Target in Human Cancers.Front Oncol. 2021 Jul 22;11:679244. doi: 10.3389/fonc.2021.679244. eCollection 2021. Front Oncol. 2021. PMID: 34367966 Free PMC article. Review.
-
Knockdown of long non-coding RNA HOTAIR reverses cisplatin resistance of ovarian cancer cells through inhibiting miR-138-5p-regulated EZH2 and SIRT1.Biol Res. 2020 Apr 29;53(1):18. doi: 10.1186/s40659-020-00286-3. Biol Res. 2020. PMID: 32349783 Free PMC article.
-
Salinomycin inhibits epigenetic modulator EZH2 to enhance death receptors in colon cancer stem cells.Epigenetics. 2021 Jan-Feb;16(2):144-161. doi: 10.1080/15592294.2020.1789270. Epub 2020 Jul 8. Epigenetics. 2021. PMID: 32635858 Free PMC article.
-
Recombinant protein TRAIL-Mu3 enhances the antitumor effects in pancreatic cancer cells by strengthening the apoptotic signaling pathway.Oncol Lett. 2021 Jun;21(6):438. doi: 10.3892/ol.2021.12699. Epub 2021 Apr 1. Oncol Lett. 2021. PMID: 33868476 Free PMC article.
References
-
- Mocan T., Matea C. T., Cojocaru I., Ilie I., Tabaran F. A., Zaharie F., Iancu C., Bartos D., and Mocan L. (2014) Photothermal treatment of human pancreatic cancer using pegylated multi-walled carbon nanotubes induces apoptosis by triggering mitochondrial membrane depolarization mechanism. J. Cancer 5, 679–688 - PMC - PubMed
-
- Mayor S. (2015) Immunotherapy improves overall survival in pancreatic cancer. Lancet Oncol. 16, e58 - PubMed
-
- Bergmann L., Maute L., Heil G., Rüssel J., Weidmann E., Köberle D., Fuxius S., Weigang-Köhler K., Aulitzky W. E., Wörmann B., Hartung G., Moritz B., Edler L., Burkholder I., Scheulen M. E., and Richly H. (2015) A prospective randomised phase-II trial with gemcitabine versus gemcitabine plus sunitinib in advanced pancreatic cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Eur. J. Cancer 51, 27–36 - PubMed
-
- Hanahan D., and Weinberg R. A. (2000) The hallmarks of cancer. Cell 100, 57–70 - PubMed
-
- Schütze S., Tchikov V., and Schneider-Brachert W. (2008) Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat. Rev. Mol. Cell Biol. 9, 655–662 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

