V̇o2 kinetics associated with moderate-intensity exercise in heart failure: impact of intrathecal fentanyl inhibition of group III/IV locomotor muscle afferents
- PMID: 28476919
- PMCID: PMC5538861
- DOI: 10.1152/ajpheart.00014.2017
V̇o2 kinetics associated with moderate-intensity exercise in heart failure: impact of intrathecal fentanyl inhibition of group III/IV locomotor muscle afferents
Abstract
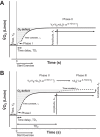
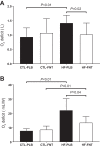
Heart failure (HF) patients demonstrate impaired pulmonary, circulatory, and nervous system responses to exercise. While HF demonstrates prolonged [time constant (τ)] pulmonary O2 uptake (V̇o2) on-kinetics, contributing to exercise intolerance, it is unknown whether abnormal V̇o2 kinetics couple with ventilatory and circulatory dysfunction secondary to impaired group III/IV afferents in HF. Because lower lumbar intrathecal fentanyl inhibits locomotor muscle afferents, resulting in improved exercise ventilation and hemodynamics, we tested these hypotheses: HF will demonstrate 1) rapid V̇o2 on-kinetics and 2) attenuated steady-state V̇o2 amplitude and O2 deficit (O2def) during exercise with fentanyl versus placebo. On separate visits (randomized), breath-by-breath V̇o2 was measured in HF (ejection fraction: 27 ± 6%, New York Heart Association class I-III) and age- and sex-matched controls (both n = 9, ages: 60 ± 6 vs. 63 ± 8 yr, P = 0.37) during cycling transitions at 65% peak workload (78 ± 24 vs. 115 ± 39 W, P < 0.01) with intrathecal fentanyl or placebo. Regardless of group or condition, optimal phase II (primary component) curve fits reflected a phase I period equal to 35 s (limb-to-lung timing) via single-exponential functions. Condition did not affect steady-state V̇o2, the phase II τ of V̇o2, or O2def within controls (P > 0.05). Without differences in steady-state V̇o2, reduced O2def in fentanyl versus placebo within HF (13 ± 4 vs. 22 ± 15 ml/W, P = 0.04) was accounted for by a rapid phase II τ of V̇o2 in fentanyl versus placebo within HF (45 ± 11 vs. 57 ± 14 s, P = 0.04), respectively. In an integrative manner, these data demonstrate important effects of abnormal locomotor muscle afferents coupled to pulmonary and circulatory dysfunction in determining impaired exercise V̇o2 in HF. Effects of abnormal muscle afferents on impaired exercise V̇o2 and hence exercise intolerance may not be discernable by independently assessing steady-state V̇o2 in HF.NEW & NOTEWORTHY Inhibition of locomotor muscle afferents results in rapid primary-component O2 uptake (V̇o2) on-kinetics accounting for the decreased O2 deficit in heart failure (HF). This study revealed that abnormal musculoskeletal-neural afferents couple with pulmonary and circulatory dysfunction to provoke impaired exercise V̇o2 in HF. Steady-state V̇o2 cannot properly phenotype abnormal muscle afferent contributions to impaired exercise V̇o2 in HF.
Keywords: exercise transition; group III-Aδ and IV-C muscle afferents; muscle oxygen uptake kinetics; on-transient oxygen uptake kinetics; oxygen deficit; square-wave exercise.
Copyright © 2017 the American Physiological Society.
Figures

Similar articles
-
Intrathecal fentanyl blockade of afferent neural feedback from skeletal muscle during exercise in heart failure patients: Influence on circulatory power and pulmonary vascular capacitance.Int J Cardiol. 2015 Dec 15;201:384-93. doi: 10.1016/j.ijcard.2015.08.101. Epub 2015 Aug 14. Int J Cardiol. 2015. PMID: 26310984 Free PMC article. Clinical Trial.
-
Locomotor muscle group III/IV afferents constrain stroke volume and contribute to exercise intolerance in human heart failure.J Physiol. 2020 Dec;598(23):5379-5390. doi: 10.1113/JP280333. Epub 2020 Sep 23. J Physiol. 2020. PMID: 32886795 Free PMC article.
-
Exercise on-transition uncoupling of ventilatory, gas exchange and cardiac hemodynamic kinetics accompany pulmonary oxygen stores depletion to impact exercise intolerance in human heart failure.Acta Physiol (Oxf). 2018 Aug;223(4):e13063. doi: 10.1111/apha.13063. Epub 2018 Apr 1. Acta Physiol (Oxf). 2018. PMID: 29575588
-
Mechanisms of the Improvement in Peak VO2 With Exercise Training in Heart Failure With Reduced or Preserved Ejection Fraction.Heart Lung Circ. 2018 Jan;27(1):9-21. doi: 10.1016/j.hlc.2017.07.002. Epub 2017 Aug 4. Heart Lung Circ. 2018. PMID: 28870770 Review.
-
Oxygen uptake kinetics during exercise.Sports Med. 1999 May;27(5):313-27. doi: 10.2165/00007256-199927050-00003. Sports Med. 1999. PMID: 10368878 Review.
Cited by
-
Contemporary Strategies to Manage High Blood Pressure in Patients with Coexistent Resistant Hypertension and Heart Failure With Reduced Ejection Fraction.Cardiol Ther. 2021 Jun;10(1):9-25. doi: 10.1007/s40119-020-00203-5. Epub 2020 Nov 17. Cardiol Ther. 2021. PMID: 33201414 Free PMC article. Review.
-
Exercise intolerance and fatigue in chronic heart failure: is there a role for group III/IV afferent feedback?Eur J Prev Cardiol. 2020 Nov;27(17):1862-1872. doi: 10.1177/2047487320906919. Epub 2020 Feb 11. Eur J Prev Cardiol. 2020. PMID: 32046526 Free PMC article.
-
Left Ventricular Assist Device Support Complicates the Exercise Physiology of Oxygen Transport and Uptake in Heart Failure.Card Fail Rev. 2019 Nov 4;5(3):162-168. doi: 10.15420/cfr.2019.10.2. eCollection 2019 Nov. Card Fail Rev. 2019. PMID: 31768273 Free PMC article. Review.
-
Metabo- and mechanoreceptor expression in human heart failure: Relationships with the locomotor muscle afferent influence on exercise responses.Exp Physiol. 2020 May;105(5):809-818. doi: 10.1113/EP088353. Epub 2020 Mar 29. Exp Physiol. 2020. PMID: 32105387 Free PMC article.
-
Statistical considerations in reporting cardiovascular research.Am J Physiol Heart Circ Physiol. 2018 Aug 1;315(2):H303-H313. doi: 10.1152/ajpheart.00309.2018. Epub 2018 Jul 20. Am J Physiol Heart Circ Physiol. 2018. PMID: 30028200 Free PMC article.
References
-
- Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research . Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122: 191–225, 2010. doi:10.1161/CIR.0b013e3181e52e69. - DOI - PubMed
-
- Barstow TJ, Molé PA. Simulation of pulmonary O2 uptake during exercise transients in humans. J Appl Physiol (1985) 63: 2253–2261, 1987. - PubMed
-
- Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 60: 2020–2027, 1986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

