Detachable glass microelectrodes for recording action potentials in active moving organs
- PMID: 28476925
- PMCID: PMC5495927
- DOI: 10.1152/ajpheart.00741.2016
Detachable glass microelectrodes for recording action potentials in active moving organs
Abstract
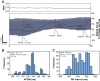
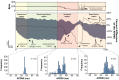
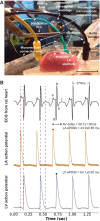
Here, we describe new detachable floating glass micropipette electrode devices that provide targeted action potential recordings in active moving organs without requiring constant mechanical constraint or pharmacological inhibition of tissue motion. The technology is based on the concept of a glass micropipette electrode that is held firmly during cell targeting and intracellular insertion, after which a 100-µg glass microelectrode, a "microdevice," is gently released to remain within the moving organ. The microdevices provide long-term recordings of action potentials, even during millimeter-scale movement of tissue in which the device is embedded. We demonstrate two different glass micropipette electrode holding and detachment designs appropriate for the heart (sharp glass microdevices for cardiac myocytes in rats, guinea pigs, and humans) and the brain (patch glass microdevices for neurons in rats). We explain how microdevices enable measurements of multiple cells within a moving organ that are typically difficult with other technologies. Using sharp microdevices, action potential duration was monitored continuously for 15 min in unconstrained perfused hearts during global ischemia-reperfusion, providing beat-to-beat measurements of changes in action potential duration. Action potentials from neurons in the hippocampus of anesthetized rats were measured with patch microdevices, which provided stable base potentials during long-term recordings. Our results demonstrate that detachable microdevices are an elegant and robust tool to record electrical activity with high temporal resolution and cellular level localization without disturbing the physiological working conditions of the organ.NEW & NOTEWORTHY Cellular action potential measurements within tissue using glass micropipette electrodes usually require tissue immobilization, potentially influencing the physiological relevance of the measurement. Here, we addressed this limitation with novel 100-µg detachable glass microelectrodes that can be precisely positioned to provide long-term measurements of action potential duration during unconstrained tissue movement.
Keywords: action potential duration; electrophysiology; glass micropipette electrodes; transmembrane potential.
Copyright © 2017 the American Physiological Society.
Figures




Similar articles
-
Application of active electrode compensation to perform continuous voltage-clamp recordings with sharp microelectrodes.J Neural Eng. 2014 Oct;11(5):056028. doi: 10.1088/1741-2560/11/5/056028. Epub 2014 Sep 23. J Neural Eng. 2014. PMID: 25246226
-
NanoTouch: intracellular recording using transmembrane conductive nanoparticles.J Neurophysiol. 2019 Nov 1;122(5):2016-2026. doi: 10.1152/jn.00359.2019. Epub 2019 Sep 4. J Neurophysiol. 2019. PMID: 31483705 Free PMC article.
-
Microelectrode arrays: a new tool to measure embryonic heart activity.J Electrocardiol. 2004;37 Suppl:104-9. doi: 10.1016/j.jelectrocard.2004.08.033. J Electrocardiol. 2004. PMID: 15534818
-
Multisite Intracellular Recordings by MEA.Adv Neurobiol. 2019;22:125-153. doi: 10.1007/978-3-030-11135-9_5. Adv Neurobiol. 2019. PMID: 31073934 Review.
-
Multi-electrode array technologies for neuroscience and cardiology.Nat Nanotechnol. 2013 Feb;8(2):83-94. doi: 10.1038/nnano.2012.265. Nat Nanotechnol. 2013. PMID: 23380931 Review.
Cited by
-
Mechanism of Action Potential Prolongation During Metabolic Inhibition in the Whole Rabbit Heart.Front Physiol. 2018 Aug 9;9:1077. doi: 10.3389/fphys.2018.01077. eCollection 2018. Front Physiol. 2018. PMID: 30140239 Free PMC article.
-
Guidelines for assessment of cardiac electrophysiology and arrhythmias in small animals.Am J Physiol Heart Circ Physiol. 2022 Dec 1;323(6):H1137-H1166. doi: 10.1152/ajpheart.00439.2022. Epub 2022 Oct 21. Am J Physiol Heart Circ Physiol. 2022. PMID: 36269644 Free PMC article. Review.
-
Ephaptic Coupling Is a Mechanism of Conduction Reserve During Reduced Gap Junction Coupling.Front Physiol. 2022 May 5;13:848019. doi: 10.3389/fphys.2022.848019. eCollection 2022. Front Physiol. 2022. PMID: 35600295 Free PMC article.
-
A Novel Wax Based Piezo Actuator for Autonomous Deep Anterior Lamellar Keratoplasty (Piezo-DALK).Rep U S. 2021 Sep-Oct;2021:757-764. doi: 10.1109/iros51168.2021.9636153. Epub 2021 Dec 16. Rep U S. 2021. PMID: 38170110 Free PMC article.
-
Statistical considerations in reporting cardiovascular research.Am J Physiol Heart Circ Physiol. 2018 Aug 1;315(2):H303-H313. doi: 10.1152/ajpheart.00309.2018. Epub 2018 Jul 20. Am J Physiol Heart Circ Physiol. 2018. PMID: 30028200 Free PMC article.
References
-
- Bosch RF, Wang Z, Li GR, Nattel S. Electrophysiological mechanisms by which hypothyroidism delays repolarization in guinea pig hearts. Am J Physiol 277: H211–H220, 1999. - PubMed
-
- Cauley E, Wang X, Dyavanapalli J, Sun K, Garrott K, Kuzmiak-Glancy S, Kay MW, Mendelowitz D. Neurotransmission to parasympathetic cardiac vagal neurons in the brain stem is altered with left ventricular hypertrophy-induced heart failure. Am J Physiol Heart Circ Physiol 309: H1281–H1287, 2015. doi: 10.1152/ajpheart.00445.2015. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

