Exploitation of the host cell ubiquitin machinery by microbial effector proteins
- PMID: 28476939
- PMCID: PMC5482977
- DOI: 10.1242/jcs.188482
Exploitation of the host cell ubiquitin machinery by microbial effector proteins
Abstract
Pathogenic bacteria are in a constant battle for survival with their host. In order to gain a competitive edge, they employ a variety of sophisticated strategies that allow them to modify conserved host cell processes in ways that favor bacterial survival and growth. Ubiquitylation, the covalent attachment of the small modifier ubiquitin to target proteins, is such a pathway. Ubiquitylation profoundly alters the fate of a myriad of cellular proteins by inducing changes in their stability or function, subcellular localization or interaction with other proteins. Given the importance of ubiquitylation in cell development, protein homeostasis and innate immunity, it is not surprising that this post-translational modification is exploited by a variety of effector proteins from microbial pathogens. Here, we highlight recent advances in our understanding of the many ways microbes take advantage of host ubiquitylation, along with some surprising deviations from the canonical theme. The lessons learned from the in-depth analyses of these host-pathogen interactions provide a fresh perspective on an ancient post-translational modification that we thought was well understood.This article is part of a Minifocus on Ubiquitin Regulation and Function. For further reading, please see related articles: 'Mechanisms of regulation and diversification of deubiquitylating enzyme function' by Pawel Leznicki and Yogesh Kulathu (J. Cell Sci.130, 1997-2006). 'Cell scientist to watch - Mads Gyrd-Hansen' (J. Cell Sci.130, 1981-1983).
Keywords: Bacterial effector; Microbial virulence; Ubiquitin.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
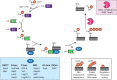
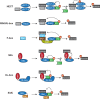




References
-
- Al-Khodor S., Price C. T., Habyarimana F., Kalia A. and Abu Kwaik Y. (2008). A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol. Microbiol. 70, 908-923. 10.1111/j.1365-2958.2008.06453.x - DOI - PMC - PubMed
-
- Angot A., Peeters N., Lechner E., Vailleau F., Baud C., Gentzbittel L., Sartorel E., Genschik P., Boucher C. and Genin S. (2006). Ralstonia solanacearum requires F-box-like domain-containing type III effectors to promote disease on several host plants. Proc. Natl. Acad. Sci. USA 103, 14620-14625. 10.1073/pnas.0509393103 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

