Impact of Age on Outcomes with Immunotherapy for Patients with Melanoma
- PMID: 28476944
- PMCID: PMC5553960
- DOI: 10.1634/theoncologist.2016-0450
Impact of Age on Outcomes with Immunotherapy for Patients with Melanoma
Abstract
Background: Monoclonal antibodies (mAb) targeting PD-1/PD-L1 have revolutionized melanoma treatment, yet data regarding effectiveness and tolerability across age groups is limited. We sought to determine the impact of age on overall survival (OS), progression-free survival (PFS), and rates of immune-mediated toxicities in patients treated with anti-PD-1/anti-PD-L1 mAb at two academic medical centers.
Methods: We retrospectively collected data on all patients with metastatic melanoma treated with anti-PD-1/PD-L1 mAb between May 2009 and April 2015. We used Kaplan-Meier and Cox regression analyses to assess OS and PFS and identify factors associated with these outcomes. We also compared rates of autoimmune toxicity across age groups.
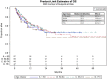
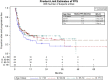
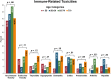
Results: Of 254 patients, 57 (22.4%) were <50 years old, 85 (33.5%) were age 50-64, 65 (25.6%) were age 65-74, and 47 (18.5%) were ≥75 years. Across age groups, no differences existed in median OS (age <50: 22.9 months, age 50-64: 25.3 months, age 65-74: 22.0 months, age ≥75: 24.3 months) or PFS (age <50: 4.1 months, age 50-64: 6.5 months, age 65-74: 5.4 months, age ≥75: 7.9 months). The presence of liver metastases and elevated pre-treatment lactate dehydrogenase (LDH) were associated with reduced OS. Presence of liver metastasis, pretreatment LDH, BRAF mutation, and type of melanoma correlated with PFS. Overall, 110 patients (43.3%) experienced immune-mediated toxicities; 25 (9.8%) had colitis and 26 (10.2%) had endocrine toxicity. Rates of colitis, hepatitis, and pneumonitis did not differ across age groups.
Conclusion: We demonstrated that patients could safely tolerate anti-PD1/PDL-1 mAb therapy and achieve similar outcomes regardless of their age.
Implications for practice: Immunotherapy has revolutionized treatment for patients with metastatic melanoma, yet data are lacking regarding the effectiveness and tolerability of these treatments for older patients. In this study, we demonstrated that patients with melanoma safely tolerate immunotherapy and achieve similar outcomes regardless of their age. Specifically, we utilized data from two academic cancer centers and found no significant difference in overall survival, progression free survival, or immune-related toxicities, other than arthritis, across age groups. As the population ages, studies such as this will become critical to help us understand how best to treat older adults with cancer.
Keywords: Geriatrics; Immunotherapy; Melanoma; Toxicity.
© AlphaMed Press 2017.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Hauschild A, Grob JJ, Demidov LV et al. Dabrafenib in BRAF‐mutated metastatic melanoma: A multicentre, open‐label, phase 3 randomised controlled trial. Lancet 2012;380:358–365. - PubMed
-
- Flaherty KT, Robert C, Hersey P et al. Improved survival with MEK inhibition in BRAF‐mutated melanoma. N Engl J Med 2012;367:107–114. - PubMed
-
- Long GV, Stroyakovskiy D, Gogas H et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877–1888. - PubMed
-
- Robert C, Karaszewska B, Schachter J et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30–39. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

