Prenatal Ethanol Exposure Persistently Alters Endocannabinoid Signaling and Endocannabinoid-Mediated Excitatory Synaptic Plasticity in Ventral Tegmental Area Dopamine Neurons
- PMID: 28476947
- PMCID: PMC5473200
- DOI: 10.1523/JNEUROSCI.3894-16.2017
Prenatal Ethanol Exposure Persistently Alters Endocannabinoid Signaling and Endocannabinoid-Mediated Excitatory Synaptic Plasticity in Ventral Tegmental Area Dopamine Neurons
Abstract
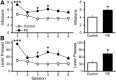
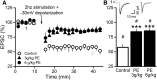
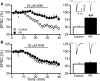
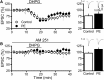
Prenatal ethanol exposure (PE) leads to increased addiction risk which could be mediated by enhanced excitatory synaptic strength in ventral tegmental area (VTA) dopamine (DA) neurons. Previous studies have shown that PE enhances excitatory synaptic strength by facilitating an anti-Hebbian form of long-term potentiation (LTP). In this study, we investigated the effect of PE on endocannabinoid-mediated long-term depression (eCB-LTD) in VTA DA neurons. Rats were exposed to moderate (3 g/kg/d) or high (6 g/kg/d) levels of ethanol during gestation. Whole-cell recordings were conducted in male offspring between 4 and 10 weeks old.We found that PE led to increased amphetamine self-administration. Both moderate and high levels of PE persistently reduced low-frequency stimulation-induced eCB-LTD. Furthermore, action potential-independent glutamate release was regulated by tonic eCB signaling in PE animals. Mechanistic studies for impaired eCB-LTD revealed that PE downregulated CB1 receptor function. Interestingly, eCB-LTD in PE animals was rescued by metabotropic glutamate receptor I activation, suggesting that PE did not impair the synthesis/release of eCBs. In contrast, eCB-LTD in PE animals was not rescued by increasing presynaptic activity, which actually led to LTP in PE animals, whereas LTD was still observed in controls. This result shows that the regulation of excitatory synaptic plasticity is fundamentally altered in PE animals. Together, PE leads to impaired eCB-LTD at the excitatory synapses of VTA DA neurons primarily due to CB1 receptor downregulation. This effect could contribute to enhanced LTP and the maintenance of augmented excitatory synaptic strength in VTA DA neurons and increased addiction risk after PE.SIGNIFICANCE STATEMENT Prenatal ethanol exposure (PE) is among many adverse developmental factors known to increase drug addiction risk. Increased excitatory synaptic strength in VTA DA neurons is a critical cellular mechanism for addiction risk. Our results show that PE persistently alters eCB signaling and impairs eCB-LTD at the excitatory synapses, an important synaptic plasticity that weakens synaptic strength. These effects combined with PE-induced anti-Hebbian long-term potentiation reported in a previous study could result in the maintenance of enhanced excitatory synaptic strength in VTA DA neurons, which in turn contributes to PE-induced increase in addiction risk. Our findings also suggest that restoring normal eCB signaling in VTA DA neurons could be a useful strategy for treating behavioral symptoms caused by PE.
Keywords: CB1 receptors; addiction risk; endocannabinoid-mediated long-term depression; long-term potentiation; synaptic homeostasis; ventral tegmental area dopamine neurons.
Copyright © 2017 the authors 0270-6474/17/375798-11$15.00/0.
Figures



Comment in
-
Commentary: Prenatal Ethanol Exposure Persistently Alters Endocannabinoid Signaling and Endocannabinioid-Mediated Excitatory Synaptic Plasticity in Ventral Tegmental Area Dopamine Neurons.Front Mol Neurosci. 2017 Nov 3;10:364. doi: 10.3389/fnmol.2017.00364. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29163039 Free PMC article. No abstract available.
Similar articles
-
Cocaine self-administration abolishes endocannabinoid-mediated long-term depression of glutamatergic synapses in the ventral tegmental area.Eur J Neurosci. 2020 Dec;52(11):4517-4524. doi: 10.1111/ejn.14980. Epub 2020 Sep 30. Eur J Neurosci. 2020. PMID: 32959420 Free PMC article.
-
Excitatory synaptic function and plasticity is persistently altered in ventral tegmental area dopamine neurons after prenatal ethanol exposure.Neuropsychopharmacology. 2015 Mar;40(4):893-905. doi: 10.1038/npp.2014.265. Epub 2014 Oct 6. Neuropsychopharmacology. 2015. PMID: 25284318 Free PMC article.
-
Neurotensin inhibits glutamate-mediated synaptic inputs onto ventral tegmental area dopamine neurons through the release of the endocannabinoid 2-AG.Neuropharmacology. 2012 Nov;63(6):983-91. doi: 10.1016/j.neuropharm.2012.07.037. Epub 2012 Jul 31. Neuropharmacology. 2012. PMID: 22884466
-
Release of endogenous cannabinoids from ventral tegmental area dopamine neurons and the modulation of synaptic processes.Prog Neuropsychopharmacol Biol Psychiatry. 2014 Jul 3;52:24-7. doi: 10.1016/j.pnpbp.2014.01.019. Epub 2014 Feb 2. Prog Neuropsychopharmacol Biol Psychiatry. 2014. PMID: 24495779 Free PMC article. Review.
-
Synaptic and intrinsic plasticity in the ventral tegmental area after chronic cocaine.Curr Opin Neurobiol. 2019 Feb;54:66-72. doi: 10.1016/j.conb.2018.08.013. Epub 2018 Sep 17. Curr Opin Neurobiol. 2019. PMID: 30237117 Free PMC article. Review.
Cited by
-
Cocaine self-administration abolishes endocannabinoid-mediated long-term depression of glutamatergic synapses in the ventral tegmental area.Eur J Neurosci. 2020 Dec;52(11):4517-4524. doi: 10.1111/ejn.14980. Epub 2020 Sep 30. Eur J Neurosci. 2020. PMID: 32959420 Free PMC article.
-
Distinct functions of endogenous cannabinoid system in alcohol abuse disorders.Br J Pharmacol. 2019 Sep;176(17):3085-3109. doi: 10.1111/bph.14780. Epub 2019 Jul 29. Br J Pharmacol. 2019. PMID: 31265740 Free PMC article. Review.
-
Environmental enrichment reverses increased addiction risk caused by prenatal ethanol exposure.Drug Alcohol Depend. 2018 Oct 1;191:343-347. doi: 10.1016/j.drugalcdep.2018.07.013. Epub 2018 Aug 25. Drug Alcohol Depend. 2018. PMID: 30176547 Free PMC article.
-
Gestational alcohol exposure disrupts cognitive function and striatal circuits in adult offspring.Nat Commun. 2020 May 22;11(1):2555. doi: 10.1038/s41467-020-16385-4. Nat Commun. 2020. PMID: 32444624 Free PMC article.
-
Combined exposure to alcohol and cannabis during development: Mechanisms and outcomes.Alcohol. 2023 Aug;110:1-13. doi: 10.1016/j.alcohol.2023.01.004. Epub 2023 Feb 3. Alcohol. 2023. PMID: 36740025 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
