Self-assembling nanoparticles encapsulating zoledronic acid inhibit mesenchymal stromal cells differentiation, migration and secretion of proangiogenic factors and their interactions with prostate cancer cells
- PMID: 28477013
- PMCID: PMC5522116
- DOI: 10.18632/oncotarget.17216
Self-assembling nanoparticles encapsulating zoledronic acid inhibit mesenchymal stromal cells differentiation, migration and secretion of proangiogenic factors and their interactions with prostate cancer cells
Abstract
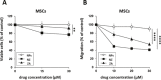
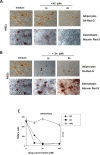
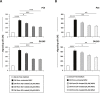
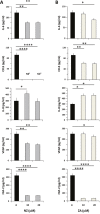
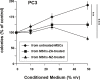
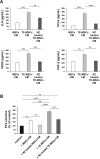
Zoledronic Acid (ZA) rapidly concentrates into the bone and reduces skeletal-related events and pain in bone metastatic prostate cancer (PCa), but exerts only a limited or absent impact as anti-cancer activity. Recently, we developed self-assembling nanoparticles (NPS) encapsulating zoledronic acid (NZ) that allowed a higher intratumor delivery of the drug compared with free zoledronic acid (ZA) in in vivo cancer models of PCa. Increasing evidence suggests that Bone Marrow (BM) Mesenchymal stromal cells (BM-MSCs) are recruited into the stroma of developing tumors where they contribute to progression by enhancing tumor growth and metastasis.We demonstrated that treatment with NZ decreased migration and differentiation into adipocytes and osteoblasts of MSCs and inhibited osteoclastogenesis. Treatment with NZ reduced the capability of MSCs to promote the migration and the clonogenic growth of the prostate cancer cell lines PC3 and DU145. The levels of Interleukin-6 and of the pro-angiogenic factors VEGF and FGF-2 were significantly reduced in MSC-CM derived from MSCs treated with NZ, and CCL5 secretion was almost totally abolished. Moreover, treatment of MSCs with supernatants from PC3 cells, leading to tumor-educated MSCs (TE-MSCs), increased the secretion of IL-6, CCL5, VEGF and FGF-2 by MSCs and increased their capability to increase PC3 cells clonogenic growth. Treatment with NZ decreased cytokine secretion and the pro-tumorigenic effects also of TE-MSCS. In conclusion, demonstrating that NZ is capable to inhibit the cross talk between MSCs and PCa, this study provides a novel insight to explain the powerful anticancer activity of NZ on PCa.
Keywords: mesenchymal stromal cells; prostate cancer; self-assembling nanoparticles; tumor microenvironment; zoledronic acid.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
References
-
- Poggi A, Musso A, Dapino I, Zocchi MR. Mechanisms of tumor escape from immune system: role of mesenchymal stromal cells. Immunol Lett. 2014;159:55–72. - PubMed
-
- Borghese C, Cattaruzza L, Pivetta E, Normanno N, De Luca A, Mazzucato M, Celegato M, Colombatti A, Aldinucci D. Gefitinib inhibits the cross-talk between mesenchymal stem cells and prostate cancer cells leading to tumor cell proliferation and inhibition of docetaxel activity. J Cell Biochem. 2013;114:1135–44. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

