Multimodal use of indocyanine green endoscopy in neurosurgery: a single-center experience and review of the literature
- PMID: 28477043
- PMCID: PMC6133047
- DOI: 10.1007/s10143-017-0858-4
Multimodal use of indocyanine green endoscopy in neurosurgery: a single-center experience and review of the literature
Abstract
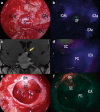
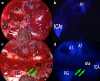
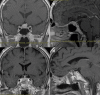
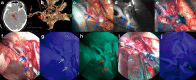
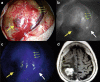
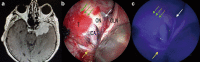
During the last 10 years, microscope-integrated indocyanine green fluorescence (m-ICG) has been widely used for assessing real-time blood flow during aneurysm surgery. More recently, an endoscope-integrated indocyanine green fluorescence (e-ICG) has been adopted as a versatile tool during different endoscopic neurosurgical procedures. The purpose of the present report is to evaluate multimodal applications of e-ICG during different endonasal, intraventricular, aneurysm and brain tumor surgeries and provide technical nuances. In addition, we reviewed the literature and identified and compare several overlapping case series of patients treated via an endoscopic integrated indocyanine green fluorescence technique. A total of 40 patients were retrospectively evaluated. Patients were divided into four main groups: (1) endoscopic endonasal approaches (n = 14); (2) ventricular endoscopic approach including patients undergoing third ventriculostomy (n = 8) and tumor biopsy (n = 1); (3) aneurysms surgery (n = 9); and (4) brain parenchymal tumors (n = 8). All patients were successfully treated using the e-ICG dynamic endoscopic visualization, and there were no perioperative complications. Such unique features open up a promising field of applications beyond the use of m-ICG in different surgical field due to the longer duration of e-ICG fluorescence up to 35 ± 7 min. E-ICG represents a new and effective technique for longer real-time visualization of vascular structures preserving normal tissues and functions during different transcranial and endonasal approaches. As the technology and e-ICG resolution improves, the technique has the potential to become a critical tool for different applications in neurosurgery.
Keywords: Aneurysm; Brain tumors; Endoscopy; Indocyanine green videoangiography; Third ventricle; Transsphenoidal.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures




References
-
- Ben-Sira I, Riva CE. Fluorescein diffusion in the human optic disc. Investig Ophthalmol. 1975;14:205–211. - PubMed
-
- Betz CS, Zhorzel S, Schachenmayr H, Stepp H, Havel M, Siedek V, Leunig A, Matthias C, Hopper C, Harreus U. Endoscopic measurements of free-flap perfusion in the head and neck region using red-excited indocyanine green: preliminary results. J Plast Reconstr Aesthet Surg. 2009;62:1602–1608. doi: 10.1016/j.bjps.2008.07.042. - DOI - PubMed
-
- Patel KM, Bhanot P, Franklin B, Albino F, Nahabedian MY. Use of intraoperative indocyanine-green angiography to minimize wound healing complications in abdominal wall reconstruction. J Plast Surg Hand Surg. 2013;47:476–480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

