Breast MRI segmentation for density estimation: Do different methods give the same results and how much do differences matter?
- PMID: 28477346
- PMCID: PMC5697622
- DOI: 10.1002/mp.12320
Breast MRI segmentation for density estimation: Do different methods give the same results and how much do differences matter?
Abstract
Purpose: To compare two methods of automatic breast segmentation with each other and with manual segmentation in a large subject cohort. To discuss the factors involved in selecting the most appropriate algorithm for automatic segmentation and, in particular, to investigate the appropriateness of overlap measures (e.g., Dice and Jaccard coefficients) as the primary determinant in algorithm selection.
Methods: Two methods of breast segmentation were applied to the task of calculating MRI breast density in 200 subjects drawn from the Avon Longitudinal Study of Parents and Children, a large cohort study with an MRI component. A semiautomated, bias-corrected, fuzzy C-means (BC-FCM) method was combined with morphological operations to segment the overall breast volume from in-phase Dixon images. The method makes use of novel, problem-specific insights. The resulting segmentation mask was then applied to the corresponding Dixon water and fat images, which were combined to give Dixon MRI density values. Contemporaneously acquired T1 - and T2 -weighted image datasets were analyzed using a novel and fully automated algorithm involving image filtering, landmark identification, and explicit location of the pectoral muscle boundary. Within the region found, fat-water discrimination was performed using an Expectation Maximization-Markov Random Field technique, yielding a second independent estimate of MRI density.
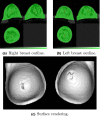
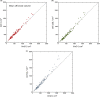
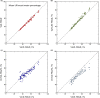
Results: Images are presented for two individual women, demonstrating how the difficulty of the problem is highly subject-specific. Dice and Jaccard coefficients comparing the semiautomated BC-FCM method, operating on Dixon source data, with expert manual segmentation are presented. The corresponding results for the method based on T1 - and T2 -weighted data are slightly lower in the individual cases shown, but scatter plots and interclass correlations for the cohort as a whole show that both methods do an excellent job in segmenting and classifying breast tissue.
Conclusions: Epidemiological results demonstrate that both methods of automated segmentation are suitable for the chosen application and that it is important to consider a range of factors when choosing a segmentation algorithm, rather than focus narrowly on a single metric such as the Dice coefficient.
Keywords: ALSPAC; MRI; breast cancer; mammographic density; segmentation.
© 2017 The Authors. Medical Physics published by Wiley Periodicals, Inc. on behalf of American Association of Physicists in Medicine.
Figures






References
-
- McCormack VA, Silva IDS. Breast density and parenchymal patterns as markers of breast cancer risk: a meta‐analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–1169. - PubMed
-
- Price ER, Keedy AW, Gidwaney R, Sickles EA, Joe BN. The potential impact of risk‐based screening mammography in women 40‐49 years old. Am J Roentgenol. 2015;205:1360–1364. - PubMed
-
- Ciatto S, Houssami N, Apruzzese A, et al. Categorizing breast mammographic density: intra‐ and interobserver reproducibility of bi‐rads density categories. Breast 2005;14:269–275. - PubMed
-
- Highnam R, Brady SM, Yaffe MJ, Karssemeijer N, Harvey J. Robust breast composition measurement ‐ volparatm In: Mart J, Oliver A, Freixenet J, Mart R, eds. Digital Mammography: 10th International Workshop, IWDM 2010, Girona, Catalonia, Spain, June 16–18, 2010. Proceedings. Berlin, Heidelberg: Springer Berlin Heidelberg; 2010: 342–349.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

