Host dihydrofolate reductase (DHFR)-directed cycloguanil analogues endowed with activity against influenza virus and respiratory syncytial virus
- PMID: 28477572
- PMCID: PMC7115580
- DOI: 10.1016/j.ejmech.2017.04.070
Host dihydrofolate reductase (DHFR)-directed cycloguanil analogues endowed with activity against influenza virus and respiratory syncytial virus
Abstract
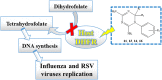
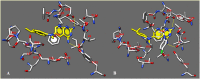
We have identified a series of 1-aryl-4,6-diamino-1,2-dihydrotriazines, structurally related to the antimalarial drug cycloguanil, as new inhibitors of influenza A and B virus and respiratory syncytial virus (RSV) via targeting of the host dihydrofolate reductase (DHFR) enzyme. Most analogues proved active against influenza B virus in the low micromolar range, and the best compounds (11, 13, 14 and 16) even reached the sub-micromolar potency of zanamivir (EC50 = 0.060 μM), and markedly exceeded (up to 327 times) the antiviral efficacy of ribavirin. Activity was also observed for two influenza A strains, including a virus with the S31N mutant form of M2 proton channel, which is the most prevalent resistance mutation for amantadine. Importantly, the compounds displayed nanomolar activity against RSV and a superior selectivity index, since the ratio of cytotoxic to antiviral concentration was >10,000 for the three most active compounds 11, 14 and 16 (EC50 ∼0.008 μM), far surpassing the potency and safety profile of the licensed drug ribavirin (EC50 = 5.8 μM, SI > 43).
Keywords: 1-Aryl-4,6-diamino-1,2-dihydrotriazine derivatives; Anti-RSV activity; Anti-influenza A and B viruses activity; Host (human) DHFR inhibition.
Copyright © 2017 Elsevier Masson SAS. All rights reserved.
Figures


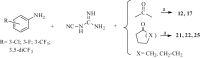


References
-
- Fineberg H.V. Pandemic preparedness and response–lessons from the H1N1 influenza of 2009. N. Engl. J. Med. 2014;370:1335–1342. - PubMed
-
- Tonelli M., Cichero E. Fight against H1N1 influenza A virus: recent insights towards the development of druggable compounds. Curr. Med. Chem. 2016;23:1802–1817. - PubMed
-
- BRaVe Research agenda . 2013. World Health Organization.http://www.who.int/influenza/patient_care/clinical/brave/en/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

