Epigallocatechin-3-gallate (EGCG) consumption in the Ts65Dn model of Down syndrome fails to improve behavioral deficits and is detrimental to skeletal phenotypes
- PMID: 28478033
- PMCID: PMC5525541
- DOI: 10.1016/j.physbeh.2017.05.003
Epigallocatechin-3-gallate (EGCG) consumption in the Ts65Dn model of Down syndrome fails to improve behavioral deficits and is detrimental to skeletal phenotypes
Abstract
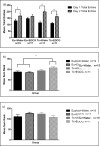
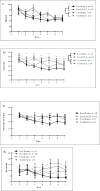
Down syndrome (DS) is caused by three copies of human chromosome 21 (Hsa21) and results in phenotypes including intellectual disability and skeletal deficits. Ts65Dn mice have three copies of ~50% of the genes homologous to Hsa21 and display phenotypes associated with DS, including cognitive deficits and skeletal abnormalities. DYRK1A is found in three copies in humans with Trisomy 21 and in Ts65Dn mice, and is involved in a number of critical pathways including neurological development and osteoclastogenesis. Epigallocatechin-3-gallate (EGCG), the main polyphenol in green tea, inhibits Dyrk1a activity. We have previously shown that EGCG treatment (~10mg/kg/day) improves skeletal abnormalities in Ts65Dn mice, yet the same dose, as well as ~20mg/kg/day did not rescue deficits in the Morris water maze spatial learning task (MWM), novel object recognition (NOR) or balance beam task (BB). In contrast, a recent study reported that an EGCG-containing supplement with a dose of 2-3mg per day (~40-60mg/kg/day) improved hippocampal-dependent task deficits in Ts65Dn mice. The current study investigated if an EGCG dosage similar to that study would yield similar improvements in either cognitive or skeletal deficits. Ts65Dn mice and euploid littermates were given EGCG [0.4mg/mL] or a water control, with treatments yielding average daily intakes of ~50mg/kg/day EGCG, and tested on the multivariate concentric square field (MCSF)-which assesses activity, exploratory behavior, risk assessment, risk taking, and shelter seeking-and NOR, BB, and MWM. EGCG treatment failed to improve cognitive deficits; EGCG also produced several detrimental effects on skeleton in both genotypes. In a refined HPLC-based assay, its first application in Ts65Dn mice, EGCG treatment significantly reduced kinase activity in femora but not in the cerebral cortex, cerebellum, or hippocampus. Counter to expectation, 9-week-old Ts65Dn mice exhibited a decrease in Dyrk1a protein levels in Western blot analysis in the cerebellum. The lack of beneficial therapeutic behavioral effects and potentially detrimental skeletal effects of EGCG found in Ts65Dn mice emphasize the importance of identifying dosages of EGCG that reliably improve DS phenotypes and linking those effects to actions of EGCG (or EGCG-containing supplements) in specific targets in brain and bone.
Keywords: Bone; Cognition; Down syndrome; EGCG; Mouse model; Trisomy 21.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

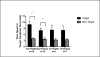
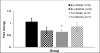

References
-
- Lejeune J, Turpin R, Gautier M. [Mongolism; a chromosomal disease (trisomy)] Bulletin de l’Academie nationale de medecine. 1959;143:256–65. - PubMed
-
- Roubertoux PL, Kerdelhue B. Trisomy 21: from chromosomes to mental retardation. Behavior genetics. 2006;36:346–54. - PubMed
-
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–16. - PubMed
-
- Van Cleve SN, Cannon S, Cohen WI. Part II: Clinical Practice Guidelines for adolescents and young adults with Down Syndrome: 12 to 21 Years. Journal of pediatric health care : official publication of National Association of Pediatric Nurse Associates & Practitioners. 2006;20:198–205. - PubMed
-
- Van Cleve SN, Cohen WI. Part I: clinical practice guidelines for children with Down syndrome from birth to 12 years. Journal of pediatric health care : official publication of National Association of Pediatric Nurse Associates & Practitioners. 2006;20:47–54. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

