Dissecting the role of myeloid and mesenchymal fibroblasts in age-dependent cardiac fibrosis
- PMID: 28478479
- PMCID: PMC5591578
- DOI: 10.1007/s00395-017-0623-4
Dissecting the role of myeloid and mesenchymal fibroblasts in age-dependent cardiac fibrosis
Abstract
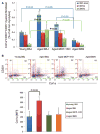
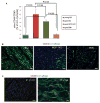
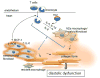
Aging is associated with increased cardiac interstitial fibrosis and diastolic dysfunction. Our previous study has shown that mesenchymal fibroblasts in the C57BL/6J (B6J) aging mouse heart acquire an inflammatory phenotype and produce higher levels of chemokines. Monocyte chemoattractant protein-1 (MCP-1) secreted by these aged fibroblasts promotes leukocyte uptake into the heart. Some of the monocytes that migrate into the heart polarize into M2a macrophages/myeloid fibroblasts. The number of activated mesenchymal fibroblasts also increases with age, and consequently, both sources of fibroblasts contribute to fibrosis. Here, we further investigate mechanisms by which inflammation influences activation of myeloid and mesenchymal fibroblasts and their collagen synthesis. We examined cardiac fibrosis and heart function in three aged mouse strains; we compared C57BL/6J (B6J) with two other strains that have reduced inflammation via different mechanisms. Aged C57BL/6N (B6N) hearts are protected from oxidative stress and fibroblasts derived from them do not develop an inflammatory phenotype. Likewise, these mice have preserved diastolic function. Aged MCP-1 null mice on the B6J background (MCP-1KO) are protected from elevated leukocyte infiltration; they develop moderate but reduced fibrosis and diastolic dysfunction. Based on these studies, we further delineated the role of resident versus monocyte-derived M2a macrophages in myeloid-dependent fibrosis and found that the number of monocyte-derived M2a (but not resident) macrophages correlates with age-related fibrosis and diastolic dysfunction. In conclusion, we have found that ROS and inflammatory mediators are necessary for activation of fibroblasts of both developmental origins, and prevention of either led to better functional outcomes.
Keywords: Aging; Fibroblast; Fibrosis; Heart; Inflammation.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures





Similar articles
-
Aicar treatment reduces interstitial fibrosis in aging mice: Suppression of the inflammatory fibroblast.J Mol Cell Cardiol. 2017 Oct;111:81-85. doi: 10.1016/j.yjmcc.2017.08.003. Epub 2017 Aug 4. J Mol Cell Cardiol. 2017. PMID: 28826664 Free PMC article.
-
Chanzyme TRPM7 protects against cardiovascular inflammation and fibrosis.Cardiovasc Res. 2020 Mar 1;116(3):721-735. doi: 10.1093/cvr/cvz164. Cardiovasc Res. 2020. PMID: 31250885 Free PMC article.
-
Mesenchymal stem cell-derived inflammatory fibroblasts promote monocyte transition into myeloid fibroblasts via an IL-6-dependent mechanism in the aging mouse heart.FASEB J. 2015 Aug;29(8):3160-70. doi: 10.1096/fj.14-268136. Epub 2015 Apr 17. FASEB J. 2015. PMID: 25888601 Free PMC article.
-
Adverse fibrosis in the aging heart depends on signaling between myeloid and mesenchymal cells; role of inflammatory fibroblasts.J Mol Cell Cardiol. 2014 May;70:56-63. doi: 10.1016/j.yjmcc.2013.10.017. Epub 2013 Oct 31. J Mol Cell Cardiol. 2014. PMID: 24184998 Free PMC article. Review.
-
Mesenchymal stem cell-derived inflammatory fibroblasts mediate interstitial fibrosis in the aging heart.J Mol Cell Cardiol. 2016 Feb;91:28-34. doi: 10.1016/j.yjmcc.2015.12.017. Epub 2015 Dec 22. J Mol Cell Cardiol. 2016. PMID: 26718722 Free PMC article. Review.
Cited by
-
Transcriptional heterogeneity of fibroblasts is a hallmark of the aging heart.JCI Insight. 2019 Nov 14;4(22):e131092. doi: 10.1172/jci.insight.131092. JCI Insight. 2019. PMID: 31723062 Free PMC article.
-
Raf kinase inhibitor protein mediates myocardial fibrosis under conditions of enhanced myocardial oxidative stress.Basic Res Cardiol. 2018 Sep 6;113(6):42. doi: 10.1007/s00395-018-0700-3. Basic Res Cardiol. 2018. PMID: 30191336 Free PMC article.
-
A defective mechanosensing pathway affects fibroblast-to-myofibroblast transition in the old male mouse heart.iScience. 2023 Jul 4;26(8):107283. doi: 10.1016/j.isci.2023.107283. eCollection 2023 Aug 18. iScience. 2023. PMID: 37520701 Free PMC article.
-
Improved Cardiovascular Function in Old Mice After N-Acetyl Cysteine and Glycine Supplemented Diet: Inflammation and Mitochondrial Factors.J Gerontol A Biol Sci Med Sci. 2018 Aug 10;73(9):1167-1177. doi: 10.1093/gerona/gly034. J Gerontol A Biol Sci Med Sci. 2018. PMID: 29538624 Free PMC article.
-
Mineralocorticoid receptor promotes cardiac macrophage inflammaging.Basic Res Cardiol. 2024 Apr;119(2):243-260. doi: 10.1007/s00395-024-01032-6. Epub 2024 Feb 8. Basic Res Cardiol. 2024. PMID: 38329499 Free PMC article.
References
-
- Arkblad EL, Tuck S, Pestov NB, Dmitriev RI, Kostina MB, Stenvall J, Tranberg M, Rydstrom J. A Caenorhabditis elegans mutant lacking functional nicotinamide nucleotide transhydrogenase displays increased sensitivity to oxidative stress. Free Radic Biol Med. 2005;38:1518–1525. doi: 10.1016/j.freeradbiomed.2005.02.012. - DOI - PubMed
-
- Burlew BS, Weber KT. Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002;27:92–98. not available. - PubMed
-
- Cardin S, Scott-Boyer MP, Praktiknjo S, Jeidane S, Picard S, Reudelhuber TL, Deschepper CF. Differences in cell-type-specific responses to angiotensin II explain cardiac remodeling differences in C57BL/6 mouse substrains. Hypertension. 2014;64:1040–1046. doi: 10.1161/HYPERTENSIONAHA.114.04067. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

