Balance of Anti-CD123 Chimeric Antigen Receptor Binding Affinity and Density for the Targeting of Acute Myeloid Leukemia
- PMID: 28479045
- PMCID: PMC5542631
- DOI: 10.1016/j.ymthe.2017.04.017
Balance of Anti-CD123 Chimeric Antigen Receptor Binding Affinity and Density for the Targeting of Acute Myeloid Leukemia
Abstract
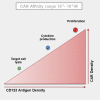
Chimeric antigen receptor (CAR)-redirected T lymphocytes are a promising immunotherapeutic approach and object of pre-clinical evaluation for the treatment of acute myeloid leukemia (AML). We developed a CAR against CD123, overexpressed on AML blasts and leukemic stem cells. However, potential recognition of low CD123-positive healthy tissues, through the on-target, off-tumor effect, limits safe clinical employment of CAR-redirected T cells. Therefore, we evaluated the effect of context-dependent variables capable of modulating CAR T cell functional profiles, such as CAR binding affinity, CAR expression, and target antigen density. Computational structural biology tools allowed for the design of rational mutations in the anti-CD123 CAR antigen binding domain that altered CAR expression and CAR binding affinity without affecting the overall CAR design. We defined both lytic and activation antigen thresholds, with early cytotoxic activity unaffected by either CAR expression or CAR affinity tuning but later effector functions impaired by low CAR expression. Moreover, the anti-CD123 CAR safety profile was confirmed by lowering CAR binding affinity, corroborating CD123 is a good therapeutic target antigen. Overall, full dissection of these variables offers suitable anti-CD123 CAR design optimization for the treatment of AML.
Keywords: AML; CAR; CD123; affinity; immunotherapy.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Brentjens R.J., Rivière I., Park J.H., Davila M.L., Wang X., Stefanski J., Taylor C., Yeh R., Bartido S., Borquez-Ojeda O. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. - PMC - PubMed
-
- Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. - PMC - PubMed
-
- Robak T., Wierzbowska A. Current and emerging therapies for acute myeloid leukemia. Clin. Ther. 2009;31:2349–2370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

