Needle adapters for intradermal administration of fractional dose of inactivated poliovirus vaccine: Evaluation of immunogenicity and programmatic feasibility in Pakistan
- PMID: 28479178
- PMCID: PMC5457301
- DOI: 10.1016/j.vaccine.2017.04.075
Needle adapters for intradermal administration of fractional dose of inactivated poliovirus vaccine: Evaluation of immunogenicity and programmatic feasibility in Pakistan
Abstract
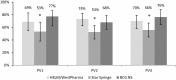
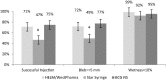
Administration of 1/5th dose of Inactivated poliovirus vaccine intradermally (fIPV) provides similar immune response as full-dose intramuscular IPV, however, fIPV administration with BCG needle and syringe (BCG NS) is technically difficult. We compared immune response after one fIPV dose administered with BCG NS to administration with intradermal devices, referred to as Device A and B; and assessed feasibility of conducting a door-to-door vaccination campaign with fIPV. In Phase I, 452 children 6-12months old from Karachi were randomized to receive one fIPV dose either with BCG NS, Device A or Device B in a health facility. Immune response was defined as seroconversion or fourfold rise in polio neutralizing antibody titer 28days after fIPV among children whose baseline titer ≤362. In Phase II, fIPV was administered during one-day door-to-door campaign to assess programmatic feasibility by evaluating vaccinators' experience. For all three poliovirus (PV) serotypes, the immune response after BCG NS and Device A was similar, however it was lower with Device B (34/44 (77%), 31/45 (69%), 16/30 (53%) respectively for PV1; 53/78 (68%), 61/83 (74%), 42/80 (53%) for PV2; and; 58/76 (76%), 56/80 (70%), 43/77 (56%) for PV3; p<0.05 for all three serotypes). Vaccinators reported problems filling Device B in both Phases; no other operational challenges were reported during Phase II. Use of fIPV offers a dose-saving alternative to full-dose IPV.
Keywords: Immune response; Inactivated polio vaccine; Intradermal injections; fractional dose IPV.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
Similar articles
-
Immune responses after fractional doses of inactivated poliovirus vaccine using newly developed intradermal jet injectors: a randomized controlled trial in Cuba.Vaccine. 2015 Jan 3;33(2):307-13. doi: 10.1016/j.vaccine.2014.11.025. Epub 2014 Nov 22. Vaccine. 2015. PMID: 25448109 Clinical Trial.
-
Immunogenicity to poliovirus type 2 following two doses of fractional intradermal inactivated poliovirus vaccine: A novel dose sparing immunization schedule.Vaccine. 2017 May 19;35(22):2993-2998. doi: 10.1016/j.vaccine.2017.03.008. Epub 2017 Apr 20. Vaccine. 2017. PMID: 28434691 Free PMC article. Review.
-
Needle-free jet injector intradermal delivery of fractional dose inactivated poliovirus vaccine: Association between injection quality and immunogenicity.Vaccine. 2015 Oct 26;33(43):5873-5877. doi: 10.1016/j.vaccine.2015.06.071. Epub 2015 Jul 17. Vaccine. 2015. PMID: 26192350 Clinical Trial.
-
Immunogenicity of full and fractional dose of inactivated poliovirus vaccine for use in routine immunisation and outbreak response: an open-label, randomised controlled trial.Lancet. 2019 Jun 29;393(10191):2624-2634. doi: 10.1016/S0140-6736(19)30503-3. Epub 2019 May 16. Lancet. 2019. PMID: 31104832 Free PMC article. Clinical Trial.
-
Equivalent schedules of intradermal fractional dose versus intramuscular full dose of inactivated polio vaccine for prevention of poliomyelitis.Cochrane Database Syst Rev. 2019 Dec 19;12(12):CD011780. doi: 10.1002/14651858.CD011780.pub2. Cochrane Database Syst Rev. 2019. PMID: 31858595 Free PMC article.
Cited by
-
Intradermal Administration of Fractional Doses of Inactivated Poliovirus Vaccine: A Dose-Sparing Option for Polio Immunization.J Infect Dis. 2017 Jul 1;216(suppl_1):S161-S167. doi: 10.1093/infdis/jix038. J Infect Dis. 2017. PMID: 28838185 Free PMC article.
-
Inactivated Poliovirus Vaccine: Recent Developments and the Tortuous Path to Global Acceptance.Pathogens. 2024 Mar 4;13(3):224. doi: 10.3390/pathogens13030224. Pathogens. 2024. PMID: 38535567 Free PMC article. Review.
-
Could Less Be More? Accounting for Fractional-Dose Regimens and Different Number of Vaccine Doses When Measuring the Impact of the RTS,S/AS01E Malaria Vaccine.J Infect Dis. 2024 Aug 16;230(2):e486-e495. doi: 10.1093/infdis/jiae075. J Infect Dis. 2024. PMID: 38438123 Free PMC article. Clinical Trial.
-
Evaluating the cost per child vaccinated with full versus fractional-dose inactivated poliovirus vaccine.Vaccine X. 2019 Jul 15;2:100032. doi: 10.1016/j.jvacx.2019.100032. eCollection 2019 Aug 9. Vaccine X. 2019. PMID: 31384747 Free PMC article.
-
An experience of mass administration of fractional dose inactivated polio vaccine through intradermal needle-free injectors in Karachi, Sindh, Pakistan.BMC Public Health. 2021 Jan 6;21(1):44. doi: 10.1186/s12889-020-10041-8. BMC Public Health. 2021. PMID: 33407294 Free PMC article.
References
-
- Cases of wild poliovirus by country and year. Available at: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Wildpoli... [accessed 12/20/2016].
-
- Sutter R.W., Platt L., Mach O., Jafari H., Aylward R.B. The new polio eradication end game: rationale and supporting evidence. J Infect Dis. 2014;210(Suppl 1):S434–S438. - PubMed
-
- Centers for Disease C, Prevention Apparent global interruption of wild poliovirus type 2 transmission. MMWR Morb Mortal Wkly Rep. 2001;50:222–224. - PubMed
-
- GPEI. Global switch in oral polio vaccines. Available at: http://maps.who.int/OPV_switch/ [accessed 07/06/2016].
-
- WHO. Outbreak response: a package of guidelines and materials. Available at: http://www.polioeradication.org/Resourcelibrary/Resourcesforpolioeradica....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

