Comprehensive Monosynaptic Rabies Virus Mapping of Host Connectivity with Neural Progenitor Grafts after Spinal Cord Injury
- PMID: 28479302
- PMCID: PMC5469919
- DOI: 10.1016/j.stemcr.2017.04.004
Comprehensive Monosynaptic Rabies Virus Mapping of Host Connectivity with Neural Progenitor Grafts after Spinal Cord Injury
Abstract
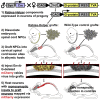
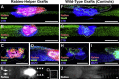
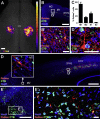
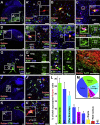
Neural progenitor cells grafted to sites of spinal cord injury have supported electrophysiological and functional recovery in several studies. Mechanisms associated with graft-related improvements in outcome appear dependent on functional synaptic integration of graft and host systems, although the extent and diversity of synaptic integration of grafts with hosts are unknown. Using transgenic mouse spinal neural progenitor cell grafts expressing the TVA and G-protein components of the modified rabies virus system, we initiated monosynaptic tracing strictly from graft neurons placed in sites of cervical spinal cord injury. We find that graft neurons receive synaptic inputs from virtually every known host system that normally innervates the spinal cord, including numerous cortical, brainstem, spinal cord, and dorsal root ganglia inputs. Thus, implanted neural progenitor cells receive an extensive range of host neural inputs to the injury site, potentially enabling functional restoration across multiple systems.
Keywords: cell therapy; grafting; monosynaptic; neural progenitor cells; neural regeneration; rabies; spinal cord injury; transsynaptic.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

