Comprehensive use of cardiac computed tomography to guide left ventricular lead placement in cardiac resynchronization therapy
- PMID: 28479514
- PMCID: PMC5575356
- DOI: 10.1016/j.hrthm.2017.04.041
Comprehensive use of cardiac computed tomography to guide left ventricular lead placement in cardiac resynchronization therapy
Abstract
Background: Optimal lead positioning is an important determinant of cardiac resynchronization therapy (CRT) response.
Objective: The purpose of this study was to evaluate cardiac computed tomography (CT) selection of the optimal epicardial vein for left ventricular (LV) lead placement by targeting regions of late mechanical activation and avoiding myocardial scar.
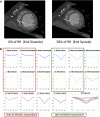
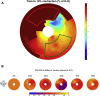
Methods: Eighteen patients undergoing CRT upgrade with existing pacing systems underwent preimplant electrocardiogram-gated cardiac CT to assess wall thickness, hypoperfusion, late mechanical activation, and regions of myocardial scar by the derivation of the stretch quantifier for endocardial engraved zones (SQUEEZ) algorithm. Cardiac venous anatomy was mapped to individualized American Heart Association (AHA) bull's-eye plots to identify the optimal venous target and compared with acute hemodynamic response (AHR) in each coronary venous target using an LV pressure wire.
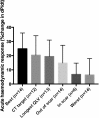
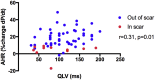
Results: Fifteen data sets were evaluable. CT-SQUEEZ-derived targets produced a similar mean AHR compared with the best achievable AHR (20.4% ± 13.7% vs 24.9% ± 11.1%; P = .36). SQUEEZ-derived guidance produced a positive AHR in 92% of target segments, and pacing in a CT-SQUEEZ target vein produced a greater clinical response rate vs nontarget segments (90% vs 60%).
Conclusion: Preprocedural CT-SQUEEZ-derived target selection may be a valuable tool to predict the optimal venous site for LV lead placement in patients undergoing CRT upgrade.
Keywords: CT guided intervention; Cardiac computed tomography; Cardiac resynchronization therapy; Dyssynchrony; Myocardial fibrosis.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Left ventricular lead positioning in cardiac resynchronization therapy: Mission accomplished?Heart Rhythm. 2017 Sep;14(9):1373-1374. doi: 10.1016/j.hrthm.2017.05.030. Epub 2017 May 24. Heart Rhythm. 2017. PMID: 28549998 No abstract available.
References
-
- Curtis A.B., Worley S.J., Adamson P.B., Chung E.S., Niazi I., Sherfesee L., Shinn T., Sutton M.S.J. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–1593. - PubMed
-
- Bleeker G.B., Kaandorp T.A., Lamb H.J., Boersma E., Steendijk P., de Roos A., Van Der Wall E.E., Schalij M.J., Bax J.J. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–976. - PubMed
-
- Ypenburg C., van Bommel R.J., Delgado V., Mollema S.A., Bleeker G.B., Boersma E., Schalij M.J., Bax J.J. Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol. 2008;52:1402–1409. - PubMed
-
- Daubert J.-C., Saxon L., Adamson P.B. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace. 2012;14:1236–1286. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

