Mn2+-coordinated PDA@DOX/PLGA nanoparticles as a smart theranostic agent for synergistic chemo-photothermal tumor therapy
- PMID: 28479854
- PMCID: PMC5411169
- DOI: 10.2147/IJN.S132270
Mn2+-coordinated PDA@DOX/PLGA nanoparticles as a smart theranostic agent for synergistic chemo-photothermal tumor therapy
Abstract
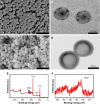
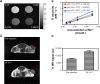
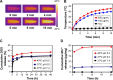
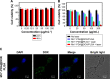
Nanoparticle drug delivery carriers, which can implement high performances of multi-functions, are of great interest, especially for improving cancer therapy. Herein, we reported a new approach to construct Mn2+-coordinated doxorubicin (DOX)-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles as a platform for synergistic chemo-photothermal tumor therapy. DOX-loaded PLGA (DOX/PLGA) nanoparticles were first synthesized through a double emulsion-solvent evaporation method, and then modified with polydopamine (PDA) through self-polymerization of dopamine, leading to the formation of PDA@DOX/PLGA nanoparticles. Mn2+ ions were then coordinated on the surfaces of PDA@DOX/PLGA to obtain Mn2+-PDA@DOX/PLGA nanoparticles. In our system, Mn2+-PDA@DOX/PLGA nanoparticles could destroy tumors in a mouse model directly, by thermal energy deposition, and could also simulate the chemotherapy by thermal-responsive delivery of DOX to enhance tumor therapy. Furthermore, the coordination of Mn2+ could afford the high magnetic resonance (MR) imaging capability with sensitivity to temperature and pH. The results demonstrated that Mn2+-PDA@ DOX/PLGA nanoparticles had a great potential as a smart theranostic agent due to their imaging and tumor-growth-inhibition properties.
Keywords: PLGA nanoparticles; chemo-photothermal therapy; polydopamine; smart theranostic agent.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Surface modification of doxorubicin-loaded nanoparticles based on polydopamine with pH-sensitive property for tumor targeting therapy.Drug Deliv. 2018 Nov;25(1):564-575. doi: 10.1080/10717544.2018.1440447. Drug Deliv. 2018. PMID: 29457518 Free PMC article.
-
Luminescent/magnetic PLGA-based hybrid nanocomposites: a smart nanocarrier system for targeted codelivery and dual-modality imaging in cancer theranostics.Int J Nanomedicine. 2017 Jun 6;12:4299-4322. doi: 10.2147/IJN.S136766. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28652734 Free PMC article.
-
A novel high drug loading mussel-inspired polydopamine hybrid nanoparticle as a pH-sensitive vehicle for drug delivery.Int J Pharm. 2017 Nov 25;533(1):73-83. doi: 10.1016/j.ijpharm.2017.09.058. Epub 2017 Sep 21. Int J Pharm. 2017. PMID: 28943209
-
Metal-Coordinated Polydopamine Structures for Tumor Imaging and Therapy.Adv Healthc Mater. 2024 Nov;13(28):e2401451. doi: 10.1002/adhm.202401451. Epub 2024 Jul 17. Adv Healthc Mater. 2024. PMID: 39021319 Review.
-
Starch-based "smart" nanomicelles: Potential delivery systems for doxorubicin.Drug Dev Res. 2024 Sep;85(6):e22253. doi: 10.1002/ddr.22253. Drug Dev Res. 2024. PMID: 39207174 Review.
Cited by
-
Polydopamine Nanosphere with In-Situ Loaded Gentamicin and Its Antimicrobial Activity.Molecules. 2020 Apr 30;25(9):2090. doi: 10.3390/molecules25092090. Molecules. 2020. PMID: 32365745 Free PMC article.
-
Smart magnetic resonance imaging-based theranostics for cancer.Theranostics. 2021 Aug 7;11(18):8706-8737. doi: 10.7150/thno.57004. eCollection 2021. Theranostics. 2021. PMID: 34522208 Free PMC article. Review.
-
Biomimetic Nanotechnology: A Natural Path Forward for Tumor-Selective and Tumor-Specific NIR Activable Photonanomedicines.Pharmaceutics. 2021 May 25;13(6):786. doi: 10.3390/pharmaceutics13060786. Pharmaceutics. 2021. PMID: 34070233 Free PMC article. Review.
-
Melanin and Melanin-Functionalized Nanoparticles as Promising Tools in Cancer Research-A Review.Cancers (Basel). 2022 Apr 6;14(7):1838. doi: 10.3390/cancers14071838. Cancers (Basel). 2022. PMID: 35406610 Free PMC article. Review.
-
Mitigating Tumor Recurrence through Mitochondrial Metabolism Inhibition: A Novel NIR Laser-Induced Therapeutic Strategy.Biol Proced Online. 2025 Jul 2;27(1):24. doi: 10.1186/s12575-025-00283-4. Biol Proced Online. 2025. PMID: 40604399 Free PMC article.
References
-
- Coates A, Abraham S, Kaye SB, et al. On the receiving end-patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Onco. 1983;19(2):203–208. - PubMed
-
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. - PubMed
-
- Brannonpeppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Delivery Rev. 2004;56(11):1649–1659. - PubMed
-
- Sher DJ, Rusthoven CC, Khan SA, Fidler MJ, Zhu H, Koshy M. National patterns of care and predictors of neoadjuvant and concurrent chemotherapy use with definitive radiotherapy in the treatment of patients with oropharyngeal squamous cell carcinoma: patterns of care for oropharyngeal cancer. Cancer. 2016;123(2):273–282. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

