Chemisorption of iodine-125 to gold nanoparticles allows for real-time quantitation and potential use in nanomedicine
- PMID: 28479864
- PMCID: PMC5397429
- DOI: 10.1007/s11051-017-3840-8
Chemisorption of iodine-125 to gold nanoparticles allows for real-time quantitation and potential use in nanomedicine
Abstract
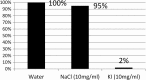
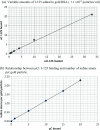
Gold nanoparticles have been available for many years as a research tool in the life sciences due to their electron density and optical properties. New applications are continually being developed, particularly in nanomedicine. One drawback is the need for an easy, real-time quantitation method for gold nanoparticles so that the effects observed in in vitro cell toxicity assays and cell uptake studies can be interpreted quantitatively in terms of nanoparticle loading. One potential method of quantifying gold nanoparticles in real time is by chemisorption of iodine-125, a gamma emitter, to the nanoparticles. This paper revisits the labelling of gold nanoparticles with iodine-125, first described 30 years ago and never fully exploited since. We explore the chemical properties and usefulness in quantifying bio-functionalised gold nanoparticle binding in a quick and simple manner. The gold particles were labelled specifically and quantitatively simply by mixing the two items. The nature of the labelling is chemisorption and is robust, remaining bound over several weeks in a variety of cell culture media. Chemisorption was confirmed as potassium iodide can remove the label whereas sodium chloride and many other buffers had no effect. Particles precoated in polymers or proteins can be labelled just as efficiently allowing for post-labelling experiments in situ rather than using radioactive gold atoms in the production process. We also demonstrate that interparticle exchange of I-125 between different size particles does not appear to take place confirming the affinity of the binding.
Keywords: Chemisorption; Gold nanoparticles; Iodine-125; Nanomedicine; Quantitation; Radioactive labelling.
Conflict of interest statement
Funding
This study was undertaken at and funded by internal funds from Liverpool University.
Conflict of interest
The author declares that he has no conflict of interest.
Figures
Similar articles
-
Temperature determination of resonantly excited plasmonic branched gold nanoparticles by X-ray absorption spectroscopy.Small. 2011 Sep 5;7(17):2498-506. doi: 10.1002/smll.201100089. Epub 2011 Jul 11. Small. 2011. PMID: 21744495
-
The gold standard: gold nanoparticle libraries to understand the nano-bio interface.Acc Chem Res. 2013 Mar 19;46(3):650-61. doi: 10.1021/ar300015b. Epub 2012 Jun 25. Acc Chem Res. 2013. PMID: 22732239
-
Radiation Nanomedicine for EGFR-Positive Breast Cancer: Panitumumab-Modified Gold Nanoparticles Complexed to the β-Particle-Emitter, (177)Lu.Mol Pharm. 2015 Nov 2;12(11):3963-72. doi: 10.1021/acs.molpharmaceut.5b00425. Epub 2015 Oct 7. Mol Pharm. 2015. PMID: 26402157
-
Gold and Silver Nanoparticles for Applications in Theranostics.Curr Top Med Chem. 2016;16(27):3069-3102. doi: 10.2174/1568026616666160715163346. Curr Top Med Chem. 2016. PMID: 27426869 Review.
-
Gold Nanoparticles: Recent Advances in the Biomedical Applications.Cell Biochem Biophys. 2015 Jul;72(3):771-5. doi: 10.1007/s12013-015-0529-4. Cell Biochem Biophys. 2015. PMID: 25663504 Review.
Cited by
-
Platinum nanoparticles labelled with iodine-125 for combined "chemo-Auger electron" therapy of hepatocellular carcinoma.Nanoscale Adv. 2023 May 12;5(12):3293-3303. doi: 10.1039/d3na00165b. eCollection 2023 Jun 13. Nanoscale Adv. 2023. PMID: 37325536 Free PMC article.
-
Harnessing Nuclear Energy to Gold Nanoparticles for the Concurrent Chemoradiotherapy of Glioblastoma.Nanomaterials (Basel). 2023 Oct 24;13(21):2821. doi: 10.3390/nano13212821. Nanomaterials (Basel). 2023. PMID: 37947667 Free PMC article.
-
Understanding the adsorption performance of hetero-nanocages (C12-B6N6, C12-Al6N6, and B6N6-Al6N6) towards hydroxyurea anticancer drug: a comprehensive study using DFT.Nanoscale Adv. 2024 Oct 3;6(23):5988-6007. doi: 10.1039/d4na00472h. Online ahead of print. Nanoscale Adv. 2024. PMID: 39372438 Free PMC article.
-
In Situ Direct Monitoring of the Morphological Transformation of Single Au Nanostars Induced by Iodide through Dual-Laser Dark-Field Microscopy: Unexpected Mechanism and Sensing Applications.Nanomaterials (Basel). 2022 Jul 25;12(15):2555. doi: 10.3390/nano12152555. Nanomaterials (Basel). 2022. PMID: 35893523 Free PMC article.
-
Methods for Radiolabelling Nanoparticles: SPECT Use (Part 1).Biomolecules. 2022 Oct 20;12(10):1522. doi: 10.3390/biom12101522. Biomolecules. 2022. PMID: 36291729 Free PMC article. Review.
References
-
- Allabashi R, Stach W, de la Escosura-Muniz A, Liste-Calleja L, Merkoci A. ICP-MS: a powerful technique for quantitative determination of gold nanoparticles without previous dissolving. J Nanopart Res. 2009;11(8):2003–2011. doi: 10.1007/s11051-008-9561-2. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
