A Cu-Catalysed Radical Cross-Dehydrogenative Coupling Approach to Acridanes and Related Heterocycles
- PMID: 28479872
- PMCID: PMC5396374
- DOI: 10.1002/ejoc.201601336
A Cu-Catalysed Radical Cross-Dehydrogenative Coupling Approach to Acridanes and Related Heterocycles
Abstract
The synthesis of acridanes and related compounds through a Cu-catalysed radical cross-dehydrogenative coupling of simple 2-[2-(arylamino)aryl]malonates is reported. This method can be further streamlined to a one-pot protocol involving the in situ fomation of the 2-[2-(arylamino)aryl]malonate by α-arylation of diethyl malonate with 2-bromodiarylamines under Pd catalysis, followed by Cu-catalysed cyclisation.
Keywords: Acridanes; Copper; Cross‐coupling; Dehydrogenation; Homogeneous catalysis; Nitrogen heterocycles; One‐pot reaction.
Figures



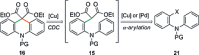
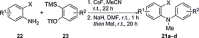
Similar articles
-
A general and mild copper-catalyzed arylation of diethyl malonate.Org Lett. 2002 Jan 24;4(2):269-72. doi: 10.1021/ol017038g. Org Lett. 2002. PMID: 11796067
-
Intermolecular Aryl C-H Amination through Sequential Iron and Copper Catalysis.Chemistry. 2017 Jan 23;23(5):1044-1047. doi: 10.1002/chem.201605671. Epub 2016 Dec 16. Chemistry. 2017. PMID: 27918637 Free PMC article.
-
Room-temperature copper-catalyzed alpha-arylation of malonates.Org Lett. 2007 Aug 16;9(17):3469-72. doi: 10.1021/ol701473p. Epub 2007 Jul 18. Org Lett. 2007. PMID: 17637034
-
Progress in copper-catalysed/mediated intramolecular dehydrogenative coupling.Org Biomol Chem. 2023 Jan 4;21(2):237-251. doi: 10.1039/d2ob01796b. Org Biomol Chem. 2023. PMID: 36448561 Review.
-
Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development.Chem Soc Rev. 2014 May 21;43(10):3525-50. doi: 10.1039/c3cs60289c. Epub 2014 Mar 3. Chem Soc Rev. 2014. PMID: 24585151 Review.
Cited by
-
DTBP-mediated cross-dehydrogenative coupling of 3-aryl benzofuran-2(3H)-ones with toluenes/phenols for all-carbon quaternary centers.RSC Adv. 2022 Dec 9;12(54):35215-35220. doi: 10.1039/d2ra06231c. eCollection 2022 Dec 6. RSC Adv. 2022. PMID: 36540229 Free PMC article.
References
-
- a) Drouhin P. and Taylor R. J. K., Eur. J. Org. Chem., 2015, 2333–2336;
- b) Hurst T. E., Gorman R. M., Drouhin P., Perry A. and Taylor R. J. K., Chem. Eur. J., 2014, 20, 14063–14073; - PubMed
- c) Klein J. E. M. N., Perry A., Pugh D. S. and Taylor R. J. K., Org. Lett., 2010, 12, 3446–3449; - PubMed
- d) Perry A. and Taylor R. J. K., Chem. Commun., 2009, 3249–3251; - PubMed
- e) Jia Y.‐X. and Kündig E. P., Angew. Chem. Int. Ed., 2009, 48, 1636–1639; - PubMed
- Angew. Chem., 2009, 121, 1664;
- f) Dey C., Larionova E. and Kündig E. P., Org. Biomol. Chem., 2013, 11, 6734–6743. - PubMed
-
- For a recent review of acridines and their derivatives, see: Schmidt A. and Liu M., Adv. Heterocycl. Chem., 2015, 115, 287–353.
-
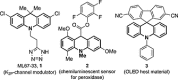
- Tessier P., Smil D. V., Wahhab A., Leit S., Rahil J., Li Z., Déziel R. and Besterman J. M., Bioorg. Med. Chem. Lett., 2009, 19, 5684–5688. - PubMed
-
- Johnson B. L., Patel M., Rodgers J. D., Tarby C. M., R. Bakthavatchalam US6593337B1, 2003.
LinkOut - more resources
Full Text Sources
Other Literature Sources
