4-Hydroxynonenal Contributes to Angiogenesis through a Redox-Dependent Sphingolipid Pathway: Prevention by Hydralazine Derivatives
- PMID: 28479957
- PMCID: PMC5396448
- DOI: 10.1155/2017/9172741
4-Hydroxynonenal Contributes to Angiogenesis through a Redox-Dependent Sphingolipid Pathway: Prevention by Hydralazine Derivatives
Abstract
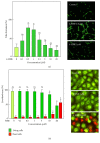
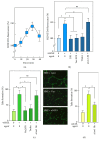
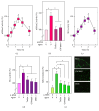
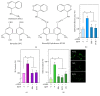
The neovascularization of atherosclerotic lesions is involved in plaque development and may contribute to intraplaque hemorrhage and plaque fragilization and rupture. Among the various proangiogenic agents involved in the neovascularization process, proatherogenic oxidized LDLs (oxLDLs) contribute to the formation of tubes via the generation of sphingosine 1-phosphate (S1P), a major mitogenic and proangiogenic sphingolipid mediator. In this study, we investigated whether 4-hydroxynonenal (4-HNE), an aldehydic lipid oxidation product abundantly present in oxLDLs, contributes to their proangiogenic properties. Immunofluorescence analysis of human atherosclerotic lesions from carotid endarterectomy showed the colocalization of HNE-adducts with CD31, a marker of endothelial cells, suggesting a close relationship between 4-HNE and neovessel formation. In vitro, low 4-HNE concentration (0.5-1 µM) elicited the formation of tubes by human microvascular endothelial cells (HMEC-1), whereas higher concentrations were not angiogenic. The formation of tubes by 4-HNE involved the generation of reactive oxygen species and the activation of the sphingolipid pathway, namely, the neutral type 2 sphingomyelinase and sphingosine kinase-1 (nSMase2/SK-1) pathway, indicating a role for S1P in the angiogenic signaling of 4-HNE. Carbonyl scavengers hydralazine and bisvanillyl-hydralazone inhibited the nSMase2/SK1 pathway activation and the formation of tubes on Matrigel® evoked by 4-HNE. Altogether, these results emphasize the role of 4-HNE in the angiogenic effect of oxLDLs and point out the potential interest of pharmacological carbonyl scavengers to prevent the neovascularization process.
Figures

Similar articles
-
The neutral sphingomyelinase-2 is involved in angiogenic signaling triggered by oxidized LDL.Free Radic Biol Med. 2016 Apr;93:204-16. doi: 10.1016/j.freeradbiomed.2016.02.006. Epub 2016 Feb 5. Free Radic Biol Med. 2016. PMID: 26855418
-
A signaling cascade mediated by ceramide, src and PDGFRβ coordinates the activation of the redox-sensitive neutral sphingomyelinase-2 and sphingosine kinase-1.Biochim Biophys Acta. 2013 Aug;1831(8):1344-56. doi: 10.1016/j.bbalip.2013.04.014. Epub 2013 May 4. Biochim Biophys Acta. 2013. PMID: 23651497
-
Proatherogenic effects of 4-hydroxynonenal.Free Radic Biol Med. 2017 Oct;111:127-139. doi: 10.1016/j.freeradbiomed.2016.12.038. Epub 2016 Dec 29. Free Radic Biol Med. 2017. PMID: 28040472 Review.
-
Antiatherogenic effect of bisvanillyl-hydralazone, a new hydralazine derivative with antioxidant, carbonyl scavenger, and antiapoptotic properties.Antioxid Redox Signal. 2011 Jun;14(11):2093-106. doi: 10.1089/ars.2010.3321. Epub 2011 Mar 13. Antioxid Redox Signal. 2011. PMID: 21043830
-
Sphingolipid signaling in renal fibrosis.Matrix Biol. 2018 Aug;68-69:230-247. doi: 10.1016/j.matbio.2018.01.006. Epub 2018 Jan 16. Matrix Biol. 2018. PMID: 29343457 Review.
Cited by
-
Cardiac-specific overexpression of aldehyde dehydrogenase 2 exacerbates cardiac remodeling in response to pressure overload.Redox Biol. 2018 Jul;17:440-449. doi: 10.1016/j.redox.2018.05.016. Epub 2018 Jun 1. Redox Biol. 2018. PMID: 29885625 Free PMC article.
-
4-Hydroxynonenal from Mitochondrial and Dietary Sources Causes Lysosomal Cell Death for Lifestyle-Related Diseases.Nutrients. 2024 Nov 30;16(23):4171. doi: 10.3390/nu16234171. Nutrients. 2024. PMID: 39683565 Free PMC article. Review.
-
Male infertility and somatic health - insights into lipid damage as a mechanistic link.Nat Rev Urol. 2022 Dec;19(12):727-750. doi: 10.1038/s41585-022-00640-y. Epub 2022 Sep 13. Nat Rev Urol. 2022. PMID: 36100661 Review.
-
Advancing Cancer Therapy with Copper/Disulfiram Nanomedicines and Drug Delivery Systems.Pharmaceutics. 2023 May 23;15(6):1567. doi: 10.3390/pharmaceutics15061567. Pharmaceutics. 2023. PMID: 37376016 Free PMC article. Review.
-
Potential Impacts of Hydralazine as a Novel Antioxidant on Cardiovascular and Renal Disease-Beyond Vasodilation and Blood Pressure Lowering.Antioxidants (Basel). 2022 Nov 11;11(11):2224. doi: 10.3390/antiox11112224. Antioxidants (Basel). 2022. PMID: 36421409 Free PMC article.
References
-
- Sueishi K., Yonemitsu Y., Nakagawa K., Kaneda Y., Kumamoto M., Nakashima Y. Atherosclerosis and angiogenesis. Its pathophysiological significance in humans as well as in an animal model induced by the gene transfer of vascular endothelial growth factor. Annals of the New York Academy of Sciences. 1997;811:311–324. doi: 10.1111/j.1749-6632.1997.tb52011.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

