Mitochondrial function and bioenergetic trade-offs during lactation in the house mouse (Mus musculus)
- PMID: 28479999
- PMCID: PMC5415517
- DOI: 10.1002/ece3.2817
Mitochondrial function and bioenergetic trade-offs during lactation in the house mouse (Mus musculus)
Abstract
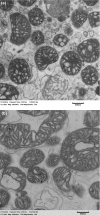
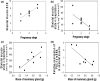
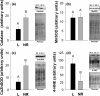
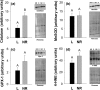
Energy allocation theory predicts that a lactating female should alter the energetic demands of its organ systems in a manner that maximizes nutrient allocation to reproduction while reducing nutrient use for tasks that are not vital to immediate survival. We posit that organ-specific plasticity in the function of mitochondria plays a key role in mediating these energetic trade-offs. The goal of this project was to evaluate mitochondrial changes that occur in response to lactation in two of the most energetically demanding organs in the body of a rodent, the liver and skeletal muscle. This work was conducted in wild-derived house mice (Mus musculus) kept in seminatural enclosures that allow the mice to maintain a natural social structure and move within a home range size typical of wild mice. Tissues were collected from females at peak lactation and from age-matched nonreproductive females. Mitochondrial respiration, oxidative damage, antioxidant, PGC-1α, and uncoupling protein levels were compared between lactating and nonreproductive females. Our findings suggest that both liver and skeletal muscle downregulate specific antioxidant proteins during lactation. The liver, but not skeletal muscle, of lactating females displayed higher oxidative damage than nonreproductive females. The liver mass of lactating females increased, but the liver displayed no change in mitochondrial respiratory control ratio. Skeletal muscle mass and mitochondrial respiratory control ratio were not different between groups. However, the respiratory function of skeletal muscle did vary among lactating females as a function of stage of concurrent pregnancy, litter size, and mass of the mammary glands. The observed changes are predicted to increase the efficiency of skeletal muscle mitochondria, reducing the substrate demands of skeletal muscle during lactation. Differences between our results and prior studies highlight the role that an animals' social and physical environment could play in how it adapts to the energetic demands of reproduction.
Keywords: antioxidants; bioenergetics; house mouse; lactation; mitochondria; oxidative damage; trade‐offs.
Figures


References
-
- Akers, R. M. (2002). Lactation and the mammary gland. Ames, IA: Iowa State University Press.
-
- Blount, J. D. , Vitikainen, E. I. K. , Stott, I. , & Cant, M. A. (2016). Oxidative shielding and the cost of reproduction. Biological Reviews, 91, 483–497. - PubMed
-
- Bradford, M. (1976a). Differential color change of a dye in response to various concentrations of protein. Analytical Biochemistry, 72, 248–253.
-
- Bradford, M. M. (1976b). Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein‐dye binding. Analytical Biochemistry, 72, 248–254. - PubMed
-
- Brambell, F. W. R. (2016). The influence of lactation on the implantation of the mammalian embryo. American Journal of Obstetrics & Gynecology, 33, 942–953.
LinkOut - more resources
Full Text Sources
Other Literature Sources

