Feasibility of common bibliometrics in evaluating translational science
- PMID: 28480055
- PMCID: PMC5408837
- DOI: 10.1017/cts.2016.8
Feasibility of common bibliometrics in evaluating translational science
Abstract
Introduction: A pilot study by 6 Clinical and Translational Science Awards (CTSAs) explored how bibliometrics can be used to assess research influence.
Methods: Evaluators from 6 institutions shared data on publications (4202 total) they supported, and conducted a combined analysis with state-of-the-art tools. This paper presents selected results based on the tools from 2 widely used vendors for bibliometrics: Thomson Reuters and Elsevier.
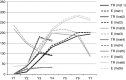
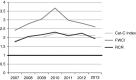
Results: Both vendors located a high percentage of publications within their proprietary databases (>90%) and provided similar but not equivalent bibliometrics for estimating productivity (number of publications) and influence (citation rates, percentage of papers in the top 10% of citations, observed citations relative to expected citations). A recently available bibliometric from the National Institutes of Health Office of Portfolio Analysis, examined after the initial analysis, showed tremendous potential for use in the CTSA context.
Conclusion: Despite challenges in making cross-CTSA comparisons, bibliometrics can enhance our understanding of the value of CTSA-supported clinical and translational research.
Keywords: CTSA; Metrics; Publications.
Figures

References
-
- National Institutes of Health. Institutional Clinical and Translational Science Award [Internet] 2008 [cited Mar 18, 2016]. (http://grants.nih.gov/grants/guide/rfa-files/RFA-RM-08-002.html)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
