Mapping the evolving definitions of translational research
- PMID: 28480056
- PMCID: PMC5408839
- DOI: 10.1017/cts.2016.10
Mapping the evolving definitions of translational research
Abstract
Objective: Systematic review and analysis of definitions of translational research.
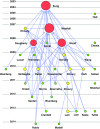
Materials and methods: The final corpus was comprised of 33 papers, each read by at least 2 reviewers. Definitions were mapped to a common set of research processes for presentation and analysis. Influence of papers and definitions was further evaluated using citation analysis and agglomerative clustering.
Results: All definitions were mapped to common research processes, revealing most common labels for each process. Agglomerative clustering revealed 3 broad families of definitions. Citation analysis showed that the originating paper of each family has been cited ~10 times more than any other member.
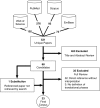
Discussion: Although there is little agreement between definitions, we were able to identify an emerging consensus 5-phase (T0-T4) definition for translational research. T1 involves processes that bring ideas from basic research through early testing in humans. T2 involves the establishment of effectiveness in humans and clinical guidelines. T3 primarily focuses on implementation and dissemination research while T4 focuses on outcomes and effectiveness in populations. T0 involves research such as genome-wide association studies which wrap back around to basic research.
Conclusion: We used systematic review and analysis to identify emerging consensus between definitions of translational research phases.
Keywords: Biomedical research/trends; Clinical trials as topic; Terminology as topic; Translational medical research; Translational medical research/trends.
Figures

References
-
- Balas EA, Boren BS. Managing clinical knowledge for health care improvement In: Bemmel J, McCray AT, eds. Yearbook of Medical Informatics 2000: Patient-Centered Systems. Stuttgart, Germany: Schattauer Verlagsgesellschaft mbH, 2000, pp. 65–70. - PubMed
-
- Institute of Medicine Clinical Research Roundtable. The Clinical Investigator Workforce: Clinical Research Roundtable Symposium I. Washington, DC: National Academy Press, 2001.
-
- Institute of Medicine Clinical Research Roundtable. Public Confidence and Involvement in Clinical Research: Clinical Research Roundtable Symposium II. Washington, DC: National Academy Press, 2001. - PubMed
-
- Institute of Medicine Clinical Research Roundtable. Exploring the Map of Clinical Research for the Coming Decade: Clinical Research Roundtable Symposium III. Washington, DC: National Academy Press, 2001. - PubMed
-
- Marwick C. Networks aim to bridge gap between clinical research, medical practice. Journal of the National Cancer Institute 2002; 94: 478–479. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
