Concurrent chemoradiation with volumetric modulated Arc therapy of patients treated for anal cancer-acute toxicity and treatment outcome
- PMID: 28480075
- PMCID: PMC5401849
- DOI: 10.21037/jgo.2017.03.09
Concurrent chemoradiation with volumetric modulated Arc therapy of patients treated for anal cancer-acute toxicity and treatment outcome
Abstract
Background: to report the acute toxicity and clinical results in patients with anal cancer treated with volumetric modulated arc therapy (VMAT) concomitant with chemotherapy.
Methods: A cohort of 21 patients with histologically confirmed squamous cell carcinoma of the anal canal was treated with VMAT and chemotherapy. Dose prescription was 39.6 Gy, 1.8 Gy/ fraction for the elective nodal PTV, the macroscopic (tumor and involved lymph nodes) PTV doses were 14.4 Gy up to a total dose of 54 Gy in 4 patients and 19.8 up to 59.4 Gy in 15 patients. One patient received a boost dose of 18 Gy up to a total dose of 57.6 Gy and another one was treated with 36 Gy and boost of 19.8 Gy up to a total dose of 55.8 Gy. Chemotherapy with MMC and 5-FU/Capecitabine was administered concomitantly. End points were local control (LC), disease-free survival (DFS) and overall survival (OS).
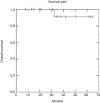
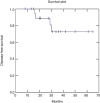
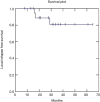
Results: Median follow-up time was 35.5 months. Two year OS was 91%, DFS was 73% and LRC was 81%. Acute dermatological toxicity G3 was recorded in one patient, ten patients (47.6%) experienced a G2 skin toxicity, while G1 toxicity was registered in eight patients (38%). One patient developed Grade 3 acute gastrointestinal (GI) toxicity, two patients (9.5%) experienced grade 2 acute GI toxicity and ten patients (47.6%) G1 toxicity. Acute genitourinary toxicity G1 was recorded in ten patients (47.6%).
Conclusions: Our results support VMAT as standard radiotherapy technique in the treatment of patients with anal cancer.
Keywords: Chemoradiation; anal cancer; results; volumetric modulated arc therapy (VMAT).
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
References
-
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Group. J Clin Oncol 1997;15:2040-9. 10.1200/JCO.1997.15.5.2040 - DOI - PubMed
-
- James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, openlabel, 2 ×2 factorial trial. Lancet Oncol 2013;14:516-24. 10.1016/S1470-2045(13)70086-X - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources