Next-generation sequencing of BRCA1/2 in breast cancer patients: potential effects on clinical decision-making using rapid, high-accuracy genetic results
- PMID: 28480178
- PMCID: PMC5416916
- DOI: 10.4174/astr.2017.92.5.331
Next-generation sequencing of BRCA1/2 in breast cancer patients: potential effects on clinical decision-making using rapid, high-accuracy genetic results
Abstract
Purpose: We evaluated the clinical role of rapid next-generation sequencing (NGS) for identifying BRCA1/2 mutations compared to traditional Sanger sequencing.
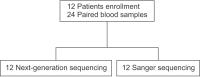
Methods: Twenty-four paired samples from 12 patients were analyzed in this prospective study to compare the performance of NGS to the Sanger method. Both NGS and Sanger sequencing were performed in 2 different laboratories using blood samples from patients with breast cancer. We then analyzed the accuracy of NGS in terms of variant calling and determining concordance rates of BRCA1/2 mutation detection.
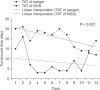
Results: The overall concordance rate of BRCA1/2 mutation identification was 100%. Variants of unknown significance (VUS) were reported in two cases of BRCA1 and 3 cases of BRCA2 after Sanger sequencing, whereas NGS reported only 1 case of BRCA1 VUS, likely due to differences in reference databases used for mutation identification. The median turnaround time of Sanger sequencing was 22 days (range, 14-26 days), while the median time of NGS was only 6 days (range, 3-21 days).
Conclusion: NGS yielded comparably accurate results to Sanger sequencing and in a much shorter time with respect to BRCA1/2 mutation identification. The shorter turnaround time and higher accuracy of NGS may help clinicians make more timely and informed decisions regarding surgery or neoadjuvant chemotherapy in patients with breast cancer.
Keywords: BRCA1; BRCA2; Breast neoplasms; High-throughput nucleotide sequencing.
Conflict of interest statement
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
Figures
References
-
- King MC, Marks JH, Mandell JB, New York Breast Cancer Study Group Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. - PubMed
-
- Wevers MR, Aaronson NK, Verhoef S, Bleiker EM, Hahn DE, Kuenen MA, et al. Impact of rapid genetic counselling and testing on the decision to undergo immediate or delayed prophylactic mastectomy in newly diagnosed breast cancer patients: findings from a randomised controlled trial. Br J Cancer. 2014;110:1081–1087. - PMC - PubMed
-
- Evans DG, Lalloo F, Ashcroft L, Shenton A, Clancy T, Baildam AD, et al. Uptake of risk-reducing surgery in unaffected women at high risk of breast and ovarian cancer is risk, age, and time dependent. Cancer Epidemiol Biomarkers Prev. 2009;18:2318–2324. - PubMed
-
- Schwartz MD, Lerman C, Brogan B, Peshkin BN, Halbert CH, DeMarco T, et al. Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. J Clin Oncol. 2004;22:1823–1829. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous