Real-World Effectiveness of Simeprevir-containing Regimens Among Patients With Chronic Hepatitis C Virus: The SONET Study
- PMID: 28480251
- PMCID: PMC5413999
- DOI: 10.1093/ofid/ofw258
Real-World Effectiveness of Simeprevir-containing Regimens Among Patients With Chronic Hepatitis C Virus: The SONET Study
Abstract
Background: The Simeprevir ObservatioNal Effectiveness across practice seTtings (SONET) study evaluated the real-world effectiveness of simeprevir-based treatment for hepatitis C virus (HCV) infection.
Methods: The SONET study was a phase 4, prospective, observational, United States-based study enrolling patients ≥18 years of age with chronic genotype 1 HCV infection. The primary endpoint was the proportion of patients who achieved sustained virologic response 12 weeks after the end of treatment (SVR12), defined as HCV ribonucleic acid undetectable ≥12 weeks after the end of all HCV treatments.
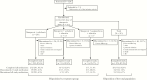
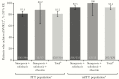
Results: Of 315 patients (intent-to-treat [ITT] population), 275 (87.3%) completed the study. Overall, 291 were treated with simeprevir + sofosbuvir, 17 with simeprevir + sofosbuvir + ribavirin, and 7 with simeprevir + peginterferon + ribavirin. The majority of patients were male (63.2%) and white (60.6%); median age was 58 years, 71.7% had genotype/subtype 1a, and 39.4% had cirrhosis. The SVR12 was achieved by 81.2% (255 of 314) of ITT patients (analysis excluded 1 patient who completed the study but was missing SVR12 data); 2 had viral breakthrough and 18 had viral relapse. The SVR12 was achieved by 92.4% (255 of 276) of patients in the modified ITT (mITT) population, which excluded patients who discontinued treatment for nonvirologic reasons before the SVR12 time point or were missing SVR12 assessment data. Among mITT patients, higher SVR12 rates were associated with factors including age ≥65 years, non-Hispanic/Latino ethnicity, and employment status, but not genotype/subtype nor presence of cirrhosis. Simeprevir-based treatment was well tolerated; no serious adverse events were considered related to simeprevir.
Conclusions: In the real-world setting, simeprevir + sofosbuvir treatment was common and 92% of mITT patients achieved SVR12. Simeprevir-based treatment was effective and well tolerated in this cohort, including patients with cirrhosis.
Keywords: hepatitis C virus; real-world; simeprevir.
The Author 2016. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- World Health Organization. Hepatitis C fact sheet number 164 Available at: http://www.who.int/mediacentre/factsheets/fs164/en/ Accessed 8 November 2016.
-
- AASLD IDSA IAS-USA. Recommendations for testing, managing, and treating hepatitis C Available at: http://www.hcvguidelines.org Accessed 18 May 2016.
-
- European Association for Study of Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol 2014; 60:392–420. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

