Humanized Mice with Subcutaneous Human Solid Tumors for Immune Response Analysis of Vaccinia Virus-Mediated Oncolysis
- PMID: 28480327
- PMCID: PMC5415323
- DOI: 10.1016/j.omto.2017.03.001
Humanized Mice with Subcutaneous Human Solid Tumors for Immune Response Analysis of Vaccinia Virus-Mediated Oncolysis
Abstract
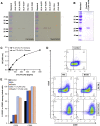
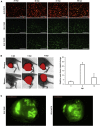
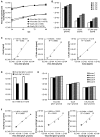
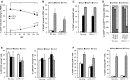
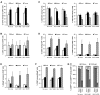
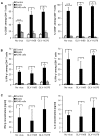
Oncolytic vaccinia virus (VACV) therapy is an alternative cancer treatment modality that mediates targeted tumor destruction through a tumor-selective replication and an induction of anti-tumor immunity. We developed a humanized tumor mouse model with subcutaneous human tumors to analyze the interactions of VACV with the developing tumors and human immune system. A successful systemic reconstitution with human immune cells including functional T cells as well as development of tumors infiltrated with human T and natural killer (NK) cells was observed. We also demonstrated successful in vivo colonization of such tumors with systemically administered VACVs. Further, a new recombinant GLV-1h376 VACV encoding for a secreted human CTLA4-blocking single-chain antibody (CTLA4 scAb) was tested. Surprisingly, although proving CTLA4 scAb's in vitro binding ability and functionality in cell culture, beside the significant increase of CD56bright NK cell subset, GLV-1h376 was not able to increase cytotoxic T or overall NK cell levels at the tumor site. Importantly, the virus-encoded β-glucuronidase as a measure of viral titer and CTLA4 scAb amount was demonstrated. Therefore, studies in our "patient-like" humanized tumor mouse model allow the exploration of newly designed therapy strategies considering the complex relationships between the developing tumor, the oncolytic virus, and the human immune system.
Figures


Similar articles
-
Viral-mediated oncolysis is the most critical factor in the late-phase of the tumor regression process upon vaccinia virus infection.BMC Cancer. 2011 Feb 14;11:68. doi: 10.1186/1471-2407-11-68. BMC Cancer. 2011. PMID: 21320309 Free PMC article.
-
Combination of fractionated irradiation with anti-VEGF expressing vaccinia virus therapy enhances tumor control by simultaneous radiosensitization of tumor associated endothelium.Int J Cancer. 2013 Dec 15;133(12):2989-99. doi: 10.1002/ijc.28296. Epub 2013 Aug 5. Int J Cancer. 2013. PMID: 23729266
-
Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus.Cancer Res. 2007 Oct 15;67(20):10038-46. doi: 10.1158/0008-5472.CAN-07-0146. Cancer Res. 2007. PMID: 17942938
-
Preferential replication of systemically delivered oncolytic vaccinia virus in focally irradiated glioma xenografts.Clin Cancer Res. 2012 May 1;18(9):2579-90. doi: 10.1158/1078-0432.CCR-11-2394. Epub 2012 Feb 29. Clin Cancer Res. 2012. PMID: 22379115
-
Preclinical Testing Oncolytic Vaccinia Virus Strain GLV-5b451 Expressing an Anti-VEGF Single-Chain Antibody for Canine Cancer Therapy.Viruses. 2015 Jul 20;7(7):4075-92. doi: 10.3390/v7072811. Viruses. 2015. PMID: 26205404 Free PMC article.
Cited by
-
White paper on microbial anti-cancer therapy and prevention.J Immunother Cancer. 2018 Aug 6;6(1):78. doi: 10.1186/s40425-018-0381-3. J Immunother Cancer. 2018. PMID: 30081947 Free PMC article. Review.
-
Cancer Immunotherapies and Humanized Mouse Drug Testing Platforms.Transl Oncol. 2019 Jul;12(7):987-995. doi: 10.1016/j.tranon.2019.04.020. Epub 2019 May 20. Transl Oncol. 2019. PMID: 31121491 Free PMC article. Review.
-
Generation of Orthotopic Patient-Derived Xenografts in Humanized Mice for Evaluation of Emerging Targeted Therapies and Immunotherapy Combinations for Melanoma.Cancers (Basel). 2023 Jul 20;15(14):3695. doi: 10.3390/cancers15143695. Cancers (Basel). 2023. PMID: 37509357 Free PMC article.
-
Gene-edited and -engineered stem cell platform drives immunotherapy for brain metastatic melanomas.Sci Transl Med. 2023 May 31;15(698):eade8732. doi: 10.1126/scitranslmed.ade8732. Epub 2023 May 31. Sci Transl Med. 2023. PMID: 37256936 Free PMC article.
-
Emerging Preclinical Applications of Humanized Mouse Models in the Discovery and Validation of Novel Immunotherapeutics and Their Mechanisms of Action for Improved Cancer Treatment.Pharmaceutics. 2023 May 26;15(6):1600. doi: 10.3390/pharmaceutics15061600. Pharmaceutics. 2023. PMID: 37376049 Free PMC article. Review.
References
-
- International Agency for Research on Cancer of World Health Organisation . World Cancer Report 2014. In: Stewart B.W., Wild C., editors. IARC; 2014.
-
- Woo Y., Adusumilli P.S., Fong Y. Advances in oncolytic viral therapy. Curr. Opin. Investig. Drugs. 2006;7:549–559. - PubMed
-
- Mullen J.T., Tanabe K.K. Viral oncolysis for malignant liver tumors. Ann. Surg. Oncol. 2003;10:596–605. - PubMed
-
- Chen N.G., Szalay A.A. Oncolytic vaccinia virus: A theranostic agent for cancer. Future Virol. 2010;5:763–784.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

