Immunological implications of pregnancy-induced microchimerism
- PMID: 28480895
- PMCID: PMC5532073
- DOI: 10.1038/nri.2017.38
Immunological implications of pregnancy-induced microchimerism
Abstract
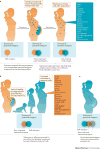
Immunological identity is traditionally defined by genetically encoded antigens, with equal maternal and paternal contributions as a result of Mendelian inheritance. However, vertically transferred maternal cells also persist in individuals at very low levels throughout postnatal development. Reciprocally, mothers are seeded during pregnancy with genetically foreign fetal cells that persist long after parturition. Recent findings suggest that these microchimeric cells expressing non-inherited, familially relevant antigenic traits are not accidental 'souvenirs' of pregnancy, but are purposefully retained within mothers and their offspring to promote genetic fitness by improving the outcome of future pregnancies. In this Review, we discuss the immunological implications, benefits and potential consequences of individuals being constitutively chimeric with a biologically active 'microchiome' of genetically foreign cells.
Conflict of interest statement
Figures


Comment in
-
Breastfeeding-related maternal microchimerism.Nat Rev Immunol. 2017 Nov;17(11):729-1. doi: 10.1038/nri.2017.115. Epub 2017 Oct 3. Nat Rev Immunol. 2017. PMID: 28972205 No abstract available.
-
Reply: Breastfeeding-related maternal microchimerism.Nat Rev Immunol. 2017 Nov;17(11):730-1. doi: 10.1038/nri.2017.117. Epub 2017 Oct 3. Nat Rev Immunol. 2017. PMID: 28972207 No abstract available.
References
-
- Medzhitov R, Janeway CA., Jr How does the immune system distinguish self from nonself? Semin. Immunol. 2000;12:185–188. discussion 257-344. - PubMed
-
- Owen RD. Immunogenetic Consequences of Vascular Anastomoses between Bovine Twins. Science. 1945;102:400–401. Pioneering description of expanded immune tolerance primed by early developmental exposure to genetically foreign antigens. - PubMed
-
- Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 1953;7:320–338.
-
- Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat. Rev. Immunol. 2013;13:23–33. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

