Inferring pathobiology from structural MRI in schizophrenia and bipolar disorder: Modeling head motion and neuroanatomical specificity
- PMID: 28480992
- PMCID: PMC6867056
- DOI: 10.1002/hbm.23612
Inferring pathobiology from structural MRI in schizophrenia and bipolar disorder: Modeling head motion and neuroanatomical specificity
Abstract
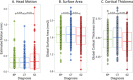
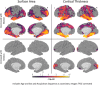
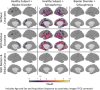
Despite over 400 peer-reviewed structural MRI publications documenting neuroanatomic abnormalities in bipolar disorder and schizophrenia, the confounding effects of head motion and the regional specificity of these defects are unclear. Using a large cohort of individuals scanned on the same research dedicated MRI with broadly similar protocols, we observe reduced cortical thickness indices in both illnesses, though less pronounced in bipolar disorder. While schizophrenia (n = 226) was associated with wide-spread surface area reductions, bipolar disorder (n = 227) and healthy comparison subjects (n = 370) did not differ. We replicate earlier reports that head motion (estimated from time-series data) influences surface area and cortical thickness measurements and demonstrate that motion influences a portion, but not all, of the observed between-group structural differences. Although the effect sizes for these differences were small to medium, when global indices were covaried during vertex-level analyses, between-group effects became nonsignificant. This analysis raises doubts about the regional specificity of structural brain changes, possible in contrast to functional changes, in affective and psychotic illnesses as measured with current imaging technology. Given that both schizophrenia and bipolar disorder showed cortical thickness reductions, but only schizophrenia showed surface area changes, and assuming these measures are influenced by at least partially unique sets of biological factors, then our results could indicate some degree of specificity between bipolar disorder and schizophrenia. Hum Brain Mapp 38:3757-3770, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: bipolar disorder; head motion; neuroanatomic specificity; neuroanatomy; neuroimaging; schizophrenia; structural MRI.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
The authors do not have financial arrangements or conflicts of interest to disclose.
Figures


References
-
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J (1998): Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: An MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry 55:663–664. - PubMed
-
- Arndt S, Cohen G, Alliger RJ, Swayze VW II, Andreasen NC (1991): Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Res 40:79–89. - PubMed
-
- Arnone D, Cavanagh J, Gerber D, Lawrie S, Ebmeier K, McIntosh A (2009): Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta‐analysis. Br J Psychiatry 195:194–201. - PubMed

