Human Neural Stem Cell Biodistribution and Predicted Tumor Coverage by a Diffusible Therapeutic in a Mouse Glioma Model
- PMID: 28481046
- PMCID: PMC5689763
- DOI: 10.1002/sctm.16-0397
Human Neural Stem Cell Biodistribution and Predicted Tumor Coverage by a Diffusible Therapeutic in a Mouse Glioma Model
Abstract
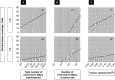
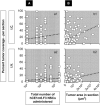
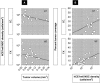
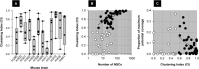
Engineered neural stem cells (NSCs) intrinsically migrating to brain tumors offer a promising mechanism for local therapeutic delivery. However, difficulties in quantitative assessments of NSC migration and in estimates of tumor coverage by diffusible therapeutics have impeded development and refinement of NSC-based therapies. To address this need, we developed techniques by which conventional serial-sectioned formalin-fixed paraffin-embedded (FFPE) brains can be analyzed in their entirety across multiple test animals. We considered a conventional human glioblastoma model: U251 glioma cells orthotopically engrafted in immunodeficient mice receiving intracerebral (i.c.) or intravenous (i.v.) administrations of NSCs expressing a diffusible enzyme to locally catalyze chemotherapeutic formation. NSC migration to tumor sites was dose-dependent, reaching 50%-60% of total administered NSCs for the i.c route and 1.5% for the i.v. route. Curiously, the most efficient NSC homing was seen with smaller NSC doses, implying existence of rate-limiting process active during administration and/or migration. Predicted tumor exposure to a diffusing therapeutic (assuming a 50 µm radius of action) could reach greater than 50% of the entire tumor volume for i.c. and 25% for i.v. administration. Within individual sections, coverage of tumor area could be as high as 100% for i.c. and 70% for i.v. routes. Greater estimated therapeutic coverage was observed for larger tumors and for larger tumor regions in individual sections. Overall, we have demonstrated a framework within which investigators may rationally evaluate NSC migration to, and integration into, brain tumors, and therefore enhance understanding of mechanisms that both promote and limit this therapeutic modality. Stem Cells Translational Medicine 2017;6:1522-1532.
Keywords: Anticancer therapy; Biodistribution; Diffusible therapeutic; Glioblastoma; Glioma; Mouse model; Neural stem cells.
© 2017 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures



References
-
- Benedetti S, Pirola B, Pollo B et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med 2000;6:447–450. - PubMed
-
- Synowitz M, Kiwit J, Kettenmann H et al. Tropism and antitumorigenic effect of endogenous neural precursors for gliomas. Clin Neurosurg 2006;53:336–344. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

