Trophoblast Glycoprotein (TPGB/5T4) in Human Placenta: Expression, Regulation, and Presence in Extracellular Microvesicles and Exosomes
- PMID: 28481180
- PMCID: PMC6343217
- DOI: 10.1177/1933719117707053
Trophoblast Glycoprotein (TPGB/5T4) in Human Placenta: Expression, Regulation, and Presence in Extracellular Microvesicles and Exosomes
Abstract
Background: Many parallels exist between growth and development of the placenta and that of cancer. One parallel is shared expression of antigens that may have functional importance and may be recognized by the immune system. Here, we characterize expression and regulation of one such antigen, Trophoblast glycoprotein (TPGB; also called 5T4), in the placenta across gestation, in placentas of preeclamptic (PE) pregnancies, and in purified microvesicles and exosomes.
Methods: Trophoblast glycoprotein expression was analyzed by real-time reverse transcription-polymerase chain reaction (RT-PCR), Western blot, and immunohistochemistry. Regulation of 5T4 in cytotrophoblast cells was examined under either differentiating conditions of epidermal growth factor or under varying oxygen conditions. Microvesicles and exosomes were purified from supernatant of cultured and perfused placentas.
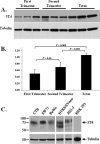
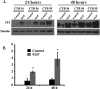
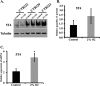
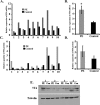
Results: Trophoblast glycoprotein expression was prominent at the microvillus surface of syncytiotrophoblast and on the extravillous trophoblast cells, with minimal expression in undifferentiated cytotrophoblasts and normal tissues. Trophoblast glycoprotein expression was elevated in malignant tumors. In cytotrophoblasts, 5T4 was induced by in vitro differentiation, and its messenger RNA (mRNA) was increased under conditions of low oxygen. PE placentas expressed higher 5T4 mRNA than matched control placentas. Trophoblast glycoprotein was prominent within shed placental microvesicles and exosomes.
Conclusion: Given the potential functional and known immunological importance of 5T4 in cancer, these studies reveal a class of proteins that may influence placental development and/or sensitize the maternal immune system. In extravillous trophoblasts, 5T4 may function in epithelial-to-mesenchymal transition during placentation. The role of syncytiotrophoblast 5T4 is unknown, but its abundance in shed syncytial vesicles may signify route of sensitization of the maternal immune system.
Keywords: 5T4; cancer; exosomes; microvesicles; placenta; trophoblast.
Conflict of interest statement
Figures



References
-
- Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblast invasion of human decidua from 8-18 weeks of pregnancy. Placenta. 1980;1(1):3–19. - PubMed
-
- Harris LK, Aplin JD. Vascular remodeling and extracellular matrix breakdown in the uterine spiral arteries during pregnancy. Reprod Sci. 2007;14(suppl 8):28–34. - PubMed
-
- Bulmer JN, Innes BA, Levey J, Robson SC, Lash GE. The role of vascular smooth muscle cell apoptosis and migration during uterine spiral artery remodeling in normal human pregnancy. FASEB J. 2012;26(7):2975–2985. - PubMed
-
- Pijnenborg R, Robertson WB, Brosnens I, Dixon G. Review article: trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 1981;2(1):71–91. - PubMed
-
- Murray MJ, Lessey BA. Embryo implantation and tumor metastasis: common pathways of invasion and angiogenesis. Semin Reprod Endocrinol. 1999;17(3):275–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

