Improved Dietary Guidelines for Vitamin D: Application of Individual Participant Data (IPD)-Level Meta-Regression Analyses
- PMID: 28481259
- PMCID: PMC5452199
- DOI: 10.3390/nu9050469
Improved Dietary Guidelines for Vitamin D: Application of Individual Participant Data (IPD)-Level Meta-Regression Analyses
Abstract
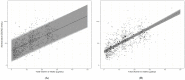
Dietary Reference Values (DRVs) for vitamin D have a key role in the prevention of vitamin D deficiency. However, despite adopting similar risk assessment protocols, estimates from authoritative agencies over the last 6 years have been diverse. This may have arisen from diverse approaches to data analysis. Modelling strategies for pooling of individual subject data from cognate vitamin D randomized controlled trials (RCTs) are likely to provide the most appropriate DRV estimates. Thus, the objective of the present work was to undertake the first-ever individual participant data (IPD)-level meta-regression, which is increasingly recognized as best practice, from seven winter-based RCTs (with 882 participants ranging in age from 4 to 90 years) of the vitamin D intake-serum 25-hydroxyvitamin D (25(OH)D) dose-response. Our IPD-derived estimates of vitamin D intakes required to maintain 97.5% of 25(OH)D concentrations >25, 30, and 50 nmol/L across the population are 10, 13, and 26 µg/day, respectively. In contrast, standard meta-regression analyses with aggregate data (as used by several agencies in recent years) from the same RCTs estimated that a vitamin D intake requirement of 14 µg/day would maintain 97.5% of 25(OH)D >50 nmol/L. These first IPD-derived estimates offer improved dietary recommendations for vitamin D because the underpinning modeling captures the between-person variability in response of serum 25(OH)D to vitamin D intake.
Keywords: DRV; EAR; Individual Participant Data-level meta-regression analyses; RDA; vitamin D recommendations.
Conflict of interest statement
The authors report no conflict of interest in this work. While Kevin D. Cashman and Susan A. Lanham-New were members of the SACN Vitamin D Working Group, and Christel Lamberg-Allardt was a member of the EFSA Vitamin D DRV panel; the findings and conclusions in this report are those of the authors and do not necessarily represent the views of these agencies. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
References
-
- Institute of Medicine Food and Nutrition Board . Dietary Reference Intakes for Calcium and Vitamin D. National Academy Press; Washington, DC, USA: 2011.
-
- Munns C.F., Shaw N., Kiely M., Specker B.L., Thacher T.D., Ozono K., Michigami T., Tiosano D., Mughal M.Z., Mäkitie O., et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. MeTable. 2016;101:394–415. doi: 10.1210/jc.2015-2175. - DOI - PMC - PubMed
-
- Cranney A., Horsley T., O’Donnell S., Weiler H., Puil L., Ooi D., Atkinson S., Ward L., Moher D., Hanley D., et al. Effectiveness and Safety of Vitamin D in Relation to Bone Health. Agency for Healthcare Research and Quality; Rockville, MD, USA: 2007. Evidence Report/Technology Assessment No. 158. - PMC - PubMed
-
- Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M., Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. MeTable. 2011;96:1911–1930. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

