Multi-Anti-Parasitic Activity of Arylidene Ketones and Thiazolidene Hydrazines against Trypanosoma cruzi and Leishmania spp
- PMID: 28481276
- PMCID: PMC6154605
- DOI: 10.3390/molecules22050709
Multi-Anti-Parasitic Activity of Arylidene Ketones and Thiazolidene Hydrazines against Trypanosoma cruzi and Leishmania spp
Abstract
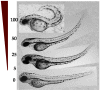
A series of fifty arylideneketones and thiazolidenehydrazines was evaluated against Leishmania infantum and Leishmania braziliensis. Furthermore, new simplified thiazolidenehydrazine derivatives were evaluated against Trypanosoma cruzi. The cytotoxicity of the active compounds on non-infected fibroblasts or macrophages was established in vitro to evaluate the selectivity of their anti-parasitic effects. Seven thiazolidenehydrazine derivatives and ten arylideneketones had good activity against the three parasites. The IC50 values for T. cruzi and Leishmania spp. ranged from 90 nM-25 µM. Eight compounds had multi-trypanocidal activity against T. cruzi and Leishmania spp. (the etiological agents of cutaneous and visceral forms). The selectivity of these active compounds was better than the three reference drugs: benznidazole, glucantime and miltefosine. They also had low toxicity when tested in vivo on zebrafish. Trying to understand the mechanism of action of these compounds, two possible molecular targets were investigated: triosephosphate isomerase and cruzipain. We also used a molecular stripping approach to elucidate the minimal structural requirements for their anti-T. cruzi activity.
Keywords: anti-T. cruzi and anti-Leishmania spp. activity; arylidene ketones; cruzipain; in vivo toxicity; thiazolidene hydrazines; triosephosphate isomerase; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- WHO . Chagas Disease (American Trypanosomiasis) WHO; Geneva, Switzerland: 2017. [(accessed on 4 May 2017)]. Available online: http://www.who.int/mediacentre/factsheets/fs340/en/
-
- Salerno R., Salvatella R., Issa J., Anzola M.C. A regional fight against Chagas disease: lessons learned from a successful collaborative partnership. Rev. Panam. De Salud Públ. 2015;37:38–43. - PubMed
-
- WHO . Weekly Epidemiological Record. Volume 21. World Health Organization; Geneva, Switzerland: 2016. pp. 421–428.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

