Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice
- PMID: 28481350
- PMCID: PMC5546121
- DOI: 10.1038/nn.4559
Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice
Abstract
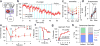
The identification of distinct cell types in the basal ganglia has been critical to our understanding of basal ganglia function and the treatment of neurological disorders. The external globus pallidus (GPe) is a key contributor to motor suppressing pathways in the basal ganglia, yet its neuronal heterogeneity has remained an untapped resource for therapeutic interventions. Here we demonstrate that optogenetic interventions that dissociate the activity of two neuronal populations in the GPe, elevating the activity of parvalbumin (PV)-expressing GPe neurons over that of Lim homeobox 6 (Lhx6)-expressing GPe neurons, restores movement in dopamine-depleted mice and attenuates pathological activity of basal ganglia output neurons for hours beyond stimulation. These results establish the utility of cell-specific interventions in the GPe to target functionally distinct pathways, with the potential to induce long-lasting recovery of movement despite the continued absence of dopamine.
Figures





Comment in
-
A cell-type-specific jolt for motor disorders.Nat Neurosci. 2017 May 25;20(6):763-765. doi: 10.1038/nn.4565. Nat Neurosci. 2017. PMID: 28542150 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

