Spectroscopic and Reactivity Comparisons of a Pair of bTAML Complexes with FeV═O and FeIV═O Units
- PMID: 28481521
- PMCID: PMC5908480
- DOI: 10.1021/acs.inorgchem.7b00448
Spectroscopic and Reactivity Comparisons of a Pair of bTAML Complexes with FeV═O and FeIV═O Units
Abstract
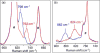
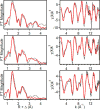
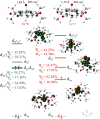
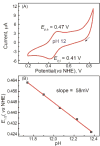
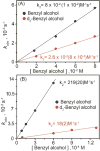
In this report we compare the geometric and electronic structures and reactivities of [FeV(O)]- and [FeIV(O)]2- species supported by the same ancillary nonheme biuret tetraamido macrocyclic ligand (bTAML). Resonance Raman studies show that the Fe═O vibration of the [FeIV(O)]2- complex 2 is at 798 cm-1, compared to 862 cm-1 for the corresponding [FeV(O)]- species 3, a 64 cm-1 frequency difference reasonably reproduced by density functional theory calculations. These values are, respectively, the lowest and the highest frequencies observed thus far for nonheme high-valent Fe═O complexes. Extended X-ray absorption fine structure analysis of 3 reveals an Fe═O bond length of 1.59 Å, which is 0.05 Å shorter than that found in complex 2. The redox potentials of 2 and 3 are 0.44 V (measured at pH 12) and 1.19 V (measured at pH 7) versus normal hydrogen electrode, respectively, corresponding to the [FeIV(O)]2-/[FeIII(OH)]2- and [FeV(O)]-/[FeIV(O)]2- couples. Consistent with its higher potential (even after correcting for the pH difference), 3 oxidizes benzyl alcohol at pH 7 with a second-order rate constant that is 2500-fold bigger than that for 2 at pH 12. Furthermore, 2 exhibits a classical kinteic isotope effect (KIE) of 3 in the oxidation of benzyl alcohol to benzaldehyde versus a nonclassical KIE of 12 for 3, emphasizing the reactivity differences between 2 and 3.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures
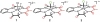


Similar articles
-
Mechanistic insights on the ortho-hydroxylation of aromatic compounds by non-heme iron complex: a computational case study on the comparative oxidative ability of ferric-hydroperoxo and high-valent Fe(IV)═O and Fe(V)═O intermediates.J Am Chem Soc. 2013 Mar 20;135(11):4235-49. doi: 10.1021/ja307077f. Epub 2013 Mar 7. J Am Chem Soc. 2013. PMID: 23373840
-
Hydrogen-bonding effects on the reactivity of [X-Fe(III)-O-Fe(IV)═O] (X = OH, F) complexes toward C-H bond cleavage.Inorg Chem. 2013 Apr 1;52(7):3976-84. doi: 10.1021/ic3027896. Epub 2013 Mar 15. Inorg Chem. 2013. PMID: 23496330 Free PMC article.
-
Kinetic Studies on the Oxoiron(IV) Complex with Tetradentate Aminopyridine Ligand PDP*: Restoration of Catalytic Activity by Reduction with H2O2.Inorg Chem. 2019 Oct 7;58(19):13382-13393. doi: 10.1021/acs.inorgchem.9b02269. Epub 2019 Sep 12. Inorg Chem. 2019. PMID: 31513388
-
Synthetic mononuclear nonheme iron-oxygen intermediates.Acc Chem Res. 2015 Aug 18;48(8):2415-23. doi: 10.1021/acs.accounts.5b00218. Epub 2015 Jul 23. Acc Chem Res. 2015. PMID: 26203519
-
Understanding the oxidative relationships of the metal oxo, hydroxo, and hydroperoxide intermediates with manganese(IV) complexes having bridged cyclams: correlation of the physicochemical properties with reactivity.Acc Chem Res. 2013 Feb 19;46(2):483-92. doi: 10.1021/ar300208z. Epub 2012 Nov 29. Acc Chem Res. 2013. PMID: 23194251
Cited by
-
A Reactive Manganese(IV)-Hydroxide Complex: A Missing Intermediate in Hydrogen Atom Transfer by High-Valent Metal-Oxo Porphyrinoid Compounds.J Am Chem Soc. 2018 Mar 28;140(12):4380-4390. doi: 10.1021/jacs.8b00350. Epub 2018 Mar 15. J Am Chem Soc. 2018. PMID: 29542921 Free PMC article.
-
Molecular catalyst coordinatively bonded to organic semiconductors for selective light-driven CO2 reduction in water.Nat Commun. 2024 Nov 12;15(1):9779. doi: 10.1038/s41467-024-54026-2. Nat Commun. 2024. PMID: 39532887 Free PMC article.
-
Closed Shell Iron(IV) Oxo Complex with an Fe-O Triple Bond: Computational Design, Synthesis, and Reactivity.Angew Chem Int Ed Engl. 2020 Dec 14;59(51):23137-23144. doi: 10.1002/anie.202009347. Epub 2020 Oct 29. Angew Chem Int Ed Engl. 2020. PMID: 32926539 Free PMC article.
-
Functional Model of Compound II of Cytochrome P450: Spectroscopic Characterization and Reactivity Studies of a FeIV-OH Complex.JACS Au. 2024 Mar 11;4(3):1142-1154. doi: 10.1021/jacsau.3c00844. eCollection 2024 Mar 25. JACS Au. 2024. PMID: 38559734 Free PMC article.
-
Stacking of a Cofacially Stacked Iron Phthalocyanine Dimer on Graphite Achieved High Catalytic CH4 Oxidation Activity Comparable to That of pMMO.JACS Au. 2023 Jan 10;3(3):823-833. doi: 10.1021/jacsau.2c00618. eCollection 2023 Mar 27. JACS Au. 2023. PMID: 37006766 Free PMC article.
References
-
- Denisov IG, Makris TM, Sligar SG, Schlichting I. Structure and Chemistry of Cytochrome P450. Chem Rev. 2005;105:2253–2278. - PubMed
- Ortiz de Montellano PR. Hydrocarbon Hydroxylation by Cyctochrome P450 Enzymes. Chem Rev. 2010;110:932–948. - PMC - PubMed
- Groves JT. Models and Mechanisms of Cytochrome P450 Action. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism and Biochemistry. 3rd. Kluwer Academic/Plenum Publishers; New York: 2005.
-
- Sono M, Roach MP, Coulter ED, Dawson JH. Heme-Containing Oxygenases. Chem Rev. 1996;1996:2841–2888. - PubMed
- Nam W. High-valent iron(IV)-oxo complexes of heme and non-heme ligands in oxygenation reactions. Acc Chem Res. 2007;40:522–531. - PubMed
- Rittle J, Green MT. Cyctochrome P450 compound I: capture, characterization, and C-H bond activation kinetics. Science. 2010;330:933–937. - PubMed
- Meunier B, de Visser SIP, Shaik S. Mechanism of Oxidation Reactions Catalyzed by Cytochrome P450 Enzymes. Chem Rev. 2004;104:3947–3980. - PubMed
- Costas M, Mehn MP, Jensen MP, Que L., Jr Dioxygen Activation at Mononuclear Non-heme Iron Active Sites: Enzymes, Models, and Intermediates. Chem Rev. 2004;104:939–986. - PubMed
- Abu-Omar MM, Loaiza A, Hontzeas N. Reaction Mechanisms of Mononuclear Non-Heme Iron Oxygenases. Chem Rev. 2005;105:2227–2252. - PubMed
-
- Groves JT, Gross Z, Stern MK. Preperation and Reactivity of Oxoiron(IV) Porphyrins. Inorg Chem. 1994;33:5065–5072.
- Nam W, Lee HJ, Oh SY, Kim C, Jang HG. First success of catalytic epoxidation of olefins by an electron-rich iron(III) porphyrin complex and H2O2: imidazole effect on the activation of H2O2 by iron porphyrin complexes in aprotic solvent. J Inorg Biochem. 2000;80:219–225. - PubMed
- Nam W, Park SE, Lim IK, Lim MH, Hong J, Kim J. First direct evidence for stereospecific olefin epoxidation and alkane hydroxylation by an oxoiron(IV) porphyrin complex. J Am Chem Soc. 2003;125:14674–14675. - PubMed
- Nehru K, Seo MS, Kim J, Nam W. Oxidative N-Dealkylation Reactions by Oxoiron(IV) Complexes of Nonheme and Heme Ligands. Inorg Chem. 2007;46:293–298. - PubMed
- Park MJ, Lee J, Suh Y, Kim J, Nam W. Reactivities of Mononuclear Non-Heme Iron Intermediates Including Evidence that Iron(III)-Hydroperoxo Species Is a Sluggish Oxidant. J Am Chem Soc. 2006;128:2630–2634. - PubMed
- Jeong YJ, Kang Y, Han AR, Lee YM, Kotani H, Fukuzumi S, Nam W. Hydrogen Atom Abstraction and Hydride Transfer reactions by Iron(IV)−Oxo Porphyrins. Angew Chem, Int Ed. 2008;47:7321–7324. - PubMed
- Fukuzumi S, Kotani H, Lee YM, Nam W. Sequential Electron-Transfer and Proton-Transfer Pathways in Hydride-Transfer reactions from Dihydronicotinamide Adenine Dinucleotide Analogues to Non-heme Oxoiron(IV) Complexes and p-Chloranil. Detection of Radical Cations of NADH Analogues in Acid-Promoted Hydride-Transfer Reactions. J Am Chem Soc. 2008;130:15134–15142. - PubMed
- Ji L, Franke A, Brindell M, Oszajca M, Zahl A, van Eldik R. Combined Experimental and Theoretical Study on the Reactivity of Compounds I and II in Horseradish Peroxidase Biomimetics. Chem – Eur J. 2014;20:14437–14450. - PubMed
- Fertinger C, Hessenauer-Ilicheva N, Franke A, van Eldik R. Direct Comparison of the reactivity of Model Complexes for Compounds 0, I, and II in Oxygenation, Hydrogen−Abstraction, and Hydride−Transfer Processes. Chem -Eur J. 2009;15:13435–13440. - PubMed
- Bell SR, Groves JT. A Highly Reactive P450 Model Compound I. J Am Chem Soc. 2009;131:9640–9641. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

