S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms
- PMID: 28481721
- PMCID: PMC5810487
- DOI: 10.1080/21505594.2017.1326438
S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms
Abstract
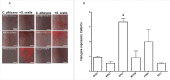
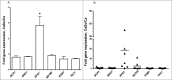
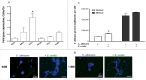
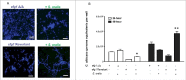
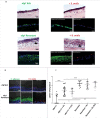
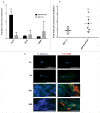
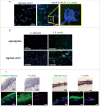
Candida albicans and Streptococcus oralis are ubiquitous oral commensal organisms. Under host-permissive conditions these organisms can form hypervirulent mucosal biofilms. C. albicans biofilm formation is controlled by 6 master transcriptional regulators: Bcr1, Brg1, Efg1, Tec1, Ndt80, and Rob1. The objective of this work was to test whether any of these regulators play a role in cross-kingdom interactions between C. albicans and S. oralis in oral mucosal biofilms, and identify downstream target gene(s) that promote these interactions. Organotypic mucosal constructs and a mouse model of oropharyngeal infection were used to analyze mucosal biofilm growth and fungal gene expression. By screening 6 C. albicans transcription regulator reporter strains we discovered that EFG1 was strongly activated by interaction with S. oralis in late biofilm growth stages. EFG1 gene expression was increased in polymicrobial biofilms on abiotic surfaces, mucosal constructs and tongue tissues of mice infected with both organisms. EFG1 was required for robust Candida-streptococcal biofilm growth in organotypic constructs and mouse oral tissues. S. oralis stimulated C. albicans ALS1 gene expression in an EFG1-dependent manner, and Als1 was identified as a downstream effector of the Efg1 pathway which promoted C. albicans-S. oralis coaggregation interactions in mixed biofilms. We conclude that S. oralis induces an increase in EFG1 expression in C. albicans in late biofilm stages. This in turn increases expression of ALS1, which promotes coaggregation interactions and mucosal biofilm growth. Our work provides novel insights on C. albicans genes which play a role in cross-kingdom interactions with S. oralis in mucosal biofilms.
Keywords: ALS1; Candida; EFG1; Streptococcus; cross-kingdom biofilm; oral mucosa.
Figures

Comment in
-
The sixth sensor: A Candida albicans biofilm master regulator that responds to inter-kingdom interactions.Virulence. 2017 Nov 17;8(8):1465-1467. doi: 10.1080/21505594.2017.1353864. Epub 2017 Aug 10. Virulence. 2017. PMID: 28700262 Free PMC article. No abstract available.
References
-
- Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, Gillevet PM. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. Plos Pathog 2010; 6:e1000713; PMID:; https://doi.org/10.1371/journal.ppat.1000713 - DOI - PMC - PubMed
-
- Dupuy AK, David MS, Li L, Heider TN, Peterson JD, Montano EA, Dongari-Bagtzoglou A, Diaz PI, Strausbaugh LD. Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: discovery of Malassezia as a prominent commensal. PLoS One 2014; 9:e90899; PMID:; https://doi.org/10.1371/journal.pone.0090899 - DOI - PMC - PubMed
-
- Xu H, Sobue T, Thompson A, Xie Z, Poon K, Ricker A, Cervantes J, Diaz PI, Dongari-Bagtzoglou A. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol 2014; 16:214-31; PMID:; https://doi.org/10.1111/cmi.12216 - DOI - PMC - PubMed
-
- Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai CH, Gonzalez-Begne M, Watson G, Krysan DJ, Bowen WH, et al.. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun 2014; 82:1968-81; PMID:; https://doi.org/10.1128/IAI.00087-14 - DOI - PMC - PubMed
-
- Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, Tuch BB, Andes DR, Johnson AD. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 2012; 148:126-38; PMID:; https://doi.org/10.1016/j.cell.2011.10.048 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
