In a randomized trial, the live attenuated tetravalent dengue vaccine TV003 is well-tolerated and highly immunogenic in subjects with flavivirus exposure prior to vaccination
- PMID: 28481883
- PMCID: PMC5436874
- DOI: 10.1371/journal.pntd.0005584
In a randomized trial, the live attenuated tetravalent dengue vaccine TV003 is well-tolerated and highly immunogenic in subjects with flavivirus exposure prior to vaccination
Abstract
Infection caused by the four serotypes of dengue virus (DENV-1-4) is a leading cause of mosquito-borne disease. Clinically-severe dengue disease is more common when secondary dengue infection occurs following prior infection with a heterologous dengue serotype. Other flaviviruses such as yellow fever virus, Japanese encephalitis virus, and Zika virus, can also elicit antibodies which are cross-reactive to DENV. As candidate dengue vaccines become available in endemic settings and for individuals who have received other flavivirus vaccines, it is important to examine vaccine safety and immunogenicity in these flavivirus-experienced populations. We performed a randomized, controlled trial of the National Institutes of Health live attenuated tetravalent dengue vaccine candidate (TV003) in fifty-eight individuals with prior exposure to flavivirus infection or vaccine. As in prior studies of this vaccine in flavivirus-naive volunteers, flavivirus-experienced subjects received two doses of vaccine six months apart and were followed closely for clinical events, laboratory changes, viremia, and neutralizing antibody titers. TV003 was well tolerated with few adverse events other than rash, which was predominately mild. Following one dose, 87% of vaccinees had an antibody response to all four serotypes (tetravalent response), suggesting a robust immune response. In addition, 76% of vaccinees were viremic; mean peak titers ranged from 0.68–1.1 log10 PFU/mL and did not differ by serotype. The second dose of TV003 was not associated with viremia, rash, or a sustained boost in antibody titers indicating that a single dose of the vaccine is likely sufficient to prevent viral replication and thus protect against disease. In comparison to the viremia and neutralizing antibody response elicited by TV003 in flavivirus-naïve subjects from prior studies, we found that subjects who were flavivirus-exposed prior to vaccination exhibited slightly higher DENV-3 viremia, higher neutralizing antibody titers to DENV-2, -3, and -4, and a higher tetravalent response frequency after TV003 administration. In summary, we demonstrate that the NIH tetravalent dengue vaccine TV003 is well-tolerated in flavivirus-experienced individuals and elicits robust post-vaccination neutralizing antibody titers.
Trial registration: ClinicalTrials.gov NCT01506570.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
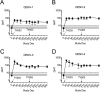
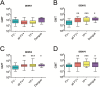
References
-
- Beatty ME, Stone A, Fitzsimons DW, Hanna JN, Lam SK, Vong S, et al. Best practices in dengue surveillance: a report from the Asia-Pacific and Americas Dengue Prevention Boards. PLoS Negl Trop Dis. 2010;4(11):e890 Epub 2010/11/26. PubMed Central PMCID: PMC2982842. doi: 10.1371/journal.pntd.0000890 - DOI - PMC - PubMed
-
- (PAHO/WHO) PAHOWHO. Five-fold increase in dengue cases in the Americas over the past decade Washington, D.C.2014, May 29. Available from: http://www.paho.org/hq/index.php?option=com_content&view=article&id=9657....
-
- Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347 PubMed Central PMCID: PMCPMC4493616. doi: 10.7554/eLife.08347 - DOI - PMC - PubMed
-
- Simmons CP, Farrar JJ, Nguyen v V, Wills B. Dengue. N Engl J Med. 2012;366(15):1423–32. Epub 2012/04/13. doi: 10.1056/NEJMra1110265 - DOI - PubMed
-
- Sabin A. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1(1):30–50. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

