Zika virus-like particle (VLP) based vaccine
- PMID: 28481898
- PMCID: PMC5436897
- DOI: 10.1371/journal.pntd.0005608
Zika virus-like particle (VLP) based vaccine
Abstract
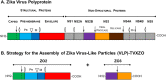
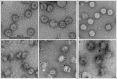
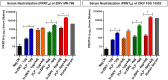
The newly emerged mosquito-borne Zika virus poses a major public challenge due to its ability to cause significant birth defects and neurological disorders. The impact of sexual transmission is unclear but raises further concerns about virus dissemination. No specific treatment or vaccine is currently available, thus the development of a safe and effective vaccine is paramount. Here we describe a novel strategy to assemble Zika virus-like particles (VLPs) by co-expressing the structural (CprME) and non-structural (NS2B/NS3) proteins, and demonstrate their effectiveness as vaccines. VLPs are produced in a suspension culture of mammalian cells and self-assembled into particles closely resembling Zika viruses as shown by electron microscopy studies. We tested various VLP vaccines and compared them to analogous compositions of an inactivated Zika virus (In-ZIKV) used as a reference. VLP immunizations elicited high titers of antibodies, as did the In-ZIKV controls. However, in mice the VLP vaccine stimulated significantly higher virus neutralizing antibody titers than comparable formulations of the In-ZIKV vaccine. The serum neutralizing activity elicited by the VLP vaccine was enhanced using a higher VLP dose and with the addition of an adjuvant, reaching neutralizing titers greater than those detected in the serum of a patient who recovered from a Zika infection in Brazil in 2015. Discrepancies in neutralization levels between the VLP vaccine and the In-ZIKV suggest that chemical inactivation has deleterious effects on neutralizing epitopes within the E protein. This along with the inability of a VLP vaccine to cause infection makes it a preferable candidate for vaccine development.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: HB and JMG are employees of TechnoVax, Inc. GRM is a TechnoVax scientific adviser and contributor. TechnoVax holds a pending patent related to this publication.
Figures





References
-
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–20. - PubMed
-
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. The New England journal of medicine. 2009;360(24):2536–43. doi: 10.1056/NEJMoa0805715 - DOI - PubMed
-
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerging infectious diseases. 2008;14(8):1232–9. PubMed Central PMCID: PMCPMC2600394. doi: 10.3201/eid1408.080287 - DOI - PMC - PubMed
-
- Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS neglected tropical diseases. 2012;6(2):e1477 PubMed Central PMCID: PMCPMC3289602. doi: 10.1371/journal.pntd.0001477 - DOI - PMC - PubMed
-
- Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, Diallo M, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS neglected tropical diseases. 2014;8(1):e2636 PubMed Central PMCID: PMCPMC3888466. doi: 10.1371/journal.pntd.0002636 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

